Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial*
framework**
Consortium leader
PETER PAZMANY CATHOLIC UNIVERSITY
Consortium members
SEMMELWEIS UNIVERSITY, DIALOG CAMPUS PUBLISHER
The Project has been realised with the support of the European Union and has been co-financed by the European Social Fund ***
**Molekuláris bionika és Infobionika Szakok tananyagának komplex fejlesztése konzorciumi keretben
***A projekt az Európai Unió támogatásával, az Európai Szociális Alap társfinanszírozásával valósul meg.
PETER PAZMANY CATHOLIC UNIVERSITY
SEMMELWEIS UNIVERSITY
Peter Pazmany Catholic University Faculty of Information Technology
MODELLING NEURONS AND NETWORKS
Lecture 6
GENESIS exercises, part 2
www.itk.ppke.hu
(Idegsejtek és neuronhálózatok modellezése)
(Második GENESIS gyakorlat)
Szabolcs Káli
Overview
In this lesson you will learn first about the GENESIS simulation environment, and use it to simulate the effects of postsynaptic potentials and explore the behavior of bursting cells.
Lesson topics:
•Simulation of postsynaptic neurons: Effects of the spatial and
temporal summation of inputs and dendritic filtering on the somatic membrane potential. Importance of the timing of inputs.
•Simulation of bursting neurons: Causes of bursting, modification of bursting patterns by modifying ion channel properties. Different
bursting patterns.
cp /usr/local/src/genesis-2.3/genesis/.simrc ~
(This command will copy the GENESIS settings to your home directory; substitute your GENESIS installation directory)
export PATH=$PATH:/usr/local/src/genesis-2.3/genesis
(This command will let you start GENESIS from any folder; this will affect the current and newly started terminals.)
Before you begin...
In a terminal window, type (if you have not done so)
Simulations of postsynaptic potentials
Start the tutorial:
cd /usr/local/src/genesis-2.3/genesis/Scripts/neuron genesis Neuron
Browse the Help menu (scan the Running the Simulation section, and look at the Neuron Inputs figure and the Model Parameters section).
Isolate the dendrites by setting RA in Dendrite 1 to 100; set all injection currents to 0, and then:
1. Look at the summation of excitatory inputs to Dendrite 1 at different input frequencies (between 20 and 1000 Hz; set the excitatory weight of Dendrite 1 to 10; to set synaptic input frequency: set all buttons to „Source A” in the dendritic input fields; in the inputs window define the input interval in
milliseconds). What makes this input excitatory?
2. Isolate Dendrite 2 by increasing its specific axial resistance to 100. How does the amplitude of a single postsynaptic potential (PSP) depend on the duration of the conductance change (you can set excitatory channel properties by pressing the Exc. Ch. button in the dendrite parameters)?
3. What happens if we add inhibition to Dendrite 1 (source B, weight 10, both sources at 500 Hz)? What happens if we also include the soma (set RA in Dendrite 1 back to 0.025)?
4. Add a cable of 25 compartments between Dendrites 1 and 2, and switch excitation to Dendrite 2 (increase its weight to 40). How does the PSP change as it travels to the soma (plot the local PSP in Dendrite 2)?
Simulations of postsynaptic potentials
5. Add an inhibitory input to dendrite 1. What is the minimal inhibitory weight needed to negate the excitatory input coming from dendrite 2 on the soma (plot the membrane potential of the soma)? Isolate Dendrite 2 by increasing its specific axial resistance to 100. How does the amplitude of the local PSP depend on the synaptic conductance (for single spikes; use the Overlay feature)?
6. Match the timing of excitatory and inhibitory input. How does the amplitude of the net PSP depend on the two weights?
7. Change the reversal potential of the inhibitory synaptic channel to -70 mV (shunting inhibition). How does the amplitude of the excitatory PSP depend on the inhibitory conductance now?
8. How does a depolarizing IPSP (reversal potential -65mV) interact with EPSPs? Is it excitatory or inhibitory?
Simulations of postsynaptic potentials
Simulations of a bursting neuron
Start the tutorial:
cd /usr/local/src/genesis-2.3/genesis/Scripts/traub91
genesis Neurokit
Load the default cell model (CA3.p) from the File menu (File -> load from file).
Browse the Help to familiarize yourself with the main features of Neurokit and to learn about the channel types shaping the response of hippocampal CA3 pyramidal neurons.
Start the simulation by pressing the „Run cell” button.
1. Place recording electrodes in the soma and the mid-apical dendrite (compartment 14); in Iclamp, set the injection current to 0.2 nA and place a current injection electrode in the soma. What do you see when you run the simulation? Can you come up with a
hypothesis about what is going on? (You might want to step through the relevant part of the simulation at 1ms steps.) How would you test your hypothesis?
Simulations of a bursting neuron
2. Click on "Show extra cell window" to also record and plot the Ca conductance and the Ca-dependent K conductance K_C (use overlay; to plot the K_C conductance, click Scale on the second cell window and type K_C into the „fieldpath” field). What could be their role in burst generation?
3. What is the role of the other Ca-dependent conductance K_AHP (doing some longer runs may be helpful)?
4. Confirm your predictions by "blocking" some of these conductances (e.g., Ca). You can do this by setting the conductance density to 0 in each compartment in the Edit cell menu. (Alternatively, you can type "setfield /CA3/##/Ca Gbar 0.0" at the GENESIS prompt.)
Simulations of a bursting neuron
Quit and restart the simulation to restore the original model.
5. How does the firing pattern change as you change the amplitude of the current injection?
6. How many qualitatively different firing patterns can you evoke? (Hint: you might also want to change the location of the injection electrode.)
Simulations of a bursting neuron
Bonus exercises
• Quit, restart, and load the CA1 pyramidal cell model "CA1.p". How does its behavior differ from that of the CA3 neuron? Explore the cause of this difference.
• Explore signal propagation in a passive cable using the "Cable"
tutorial (/usr/local/src/genesis-2.3/genesis/Scripts/cable).
• Explore the generation of pacemaker activity in simple networks using the „CPG” tutorial (/usr/local/src/genesis-
or
Neurokit tutorial 1. Recording and clamping
Recording membrane potential:
•Select the „Record” button from the Simulation control panel
•Left-click on a compartment to add a recording electrode.
Injecting current into a compartment:
•Select „IClamp” from the Simulation control panel.
•Specify the injection current in the „inject (nanoAmps)” field. In a biophysically realistic simulation the injection current should be between -5 and +5 nanoamperes.
•Left-click on a compartment to inject the current.
You can remove a recording or injection electrode by middle-clicking on the compartment. Only the type specified in the Simulation control panel will be removed.
Neurokit tutorial 2. Running the simulation
To run the simulation press „Run”
This command will run the simulation from current_time.
To display everything in the plot, click on the plot window and press
„a”.
The reset button resets the time to 0, and clears the plots. If you want to keep the plots toggle the „Do not overlay” button in the simulation graph window.
Solutions for the Neuron simulation
Exercise 1: Setup for 1000/5 = 200Hz input to Dendrite 1
Solutions for the Neuron simulation
Solutions for the Neuron simulation
Exercise 3:inhibitory and excitatory inputs (low axial resistance)
Solutions for the Neuron simulation
Exercise 5: An inhibitory input with weight 15 negates the distal excitatory input.
Solutions for the Neuron simulation
Solutions for the Neuron simulation
Solutions for the Neuron simulation
Exercise 6: Synchronous excitatory and inhibitory inputs. The excitatory weight is fixed at 15, the inhibitory weight takes the values 0, 20, 40,
Solutions for the Neuron simulation
Exercise 7: -70mV reversal potential for the inhibitory input (shunting).
Solutions for the Neuron simulation
Exercise 8: -65mV reversal potential for the inhibitory input. The inhibitory and excitatory synaptic strengths are both 15. Inhibition becomes hyperpolarizing above its reversal potential.
Solutions for the Neuron simulation
Solutions for the bursting cell simulation
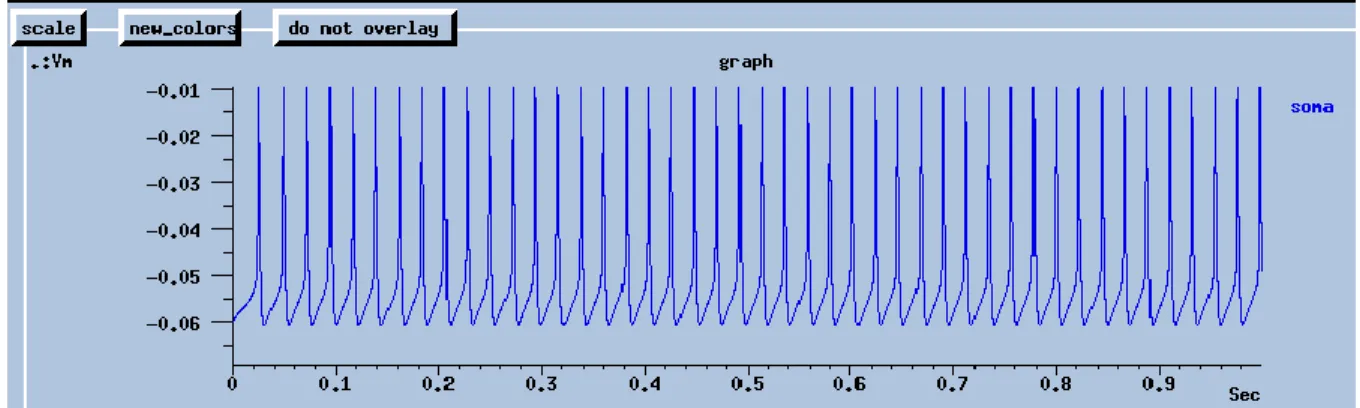
Exercise 1: plots for the hippocampal CA3 pyramidal neuron with 0.2 nA current injection into the soma (blue trace: soma; red trace: dendrite)
Solutions for the bursting cell simulation
Solutions for the bursting cell simulation
Exercise 3:
Top plot: K_AHP conductance in the soma.
Bottom plot: Somatic membrane potential.
Solutions for the bursting cell simulation Exercise 4:
Figure 1: With Ca2+ channels blocked, there is no bursting behavior
Solutions for the bursting cell simulation
Exercises 5 and 6: somatic current injections of varying strength cause qualitatively different firing patterns.
Current injection:
0.7nA 0.5nA 0.3nA 0.1nA
Solutions for the bursting cell simulation
Summary In this lesson:
• First we ran a series of postsynaptic potential simulations:
• We modeled the summation of synaptic inputs
• We analyzed the effects of extended dendrites on the propagation and summation of synaptic potentials.
• We looked at the summation of inhibitory and excitatory inputs.
• It can be concluded that both spatial and temporal summation of inputs depends on many factors, and is nonlinear even in a simple model.
• Next we investigated a bursting CA3 pyramidal cell to analyze complex spiking patterns:
• We analyzed the roles of various types of channels in bursting
behavior, and found that Ca channels are responsible for generating bursts, and Ca-dependent K channels are responsible for the pauses between bursts.
• We found that by varying the amplitude and location of the input, we can evoke qualitatively different bursting patterns.