1
Supporting Information
Conventional or mechanochemically-aided intercalation of diclofenac and naproxen anions into the interlamellar space of CaFe-layered double hydroxides and their application as dermal drug delivery systems †
Márton Szabados,
a,bAttila Gácsi,
b,cYvette Gulyás,
bZoltán Kónya,
d,eÁkos Kukovecz,
dErzsébet Csányi,
cIstván Pálinkó
a,band Pál Sipos
b,f*aDepartment of Organic Chemistry, University of Szeged, Dóm tér 8, Szeged, H-6720 Hungary
bMaterial and Solution Structure Research Group, Institute of Chemistry, University of Szeged, Aradi vértanúk tere 1, Szeged, H-6720 Hungary
cInstitute of Pharmaceutical Technology and Regulatory Affairs, University of Szeged, Eötvös utca 6, Szeged, H-6720 Hungary
dDepartment of Applied and Environmental Chemistry, University of Szeged, Rerrich B. tér 1, Szeged, H-6720 Hungary
eMTA-SZTE Reaction Kinetics and Surface Chemistry Research Group, Rerrich B. tér 1, Szeged, H-6720 Hungary
fDepartment of Inorganic and Analytical Chemistry, University of Szeged, Dóm tér 7, Szeged, H-6720 Hungary
Table S1
Comparative literature for drug release of Mg- and Zn-based LDH solids summarizing the applied kinetic models, n release exponents, and R2 linear correlation coefficients.
Investigated
nanocomposites Intercalation method Kinetic models n R2 References MgAl-LDH−naproxen Co-precipitation Korsmeyer–Peppas 0.9 0.997 Rojas et al., 2014 MgAl-LDH−naproxen Co-precipitation Korsmeyer–Peppas 0.89 0.996 Carriazo et al., 2010 MgAl-LDH−diclofenac /
Eudragit Direct anion exchange Higuchi 0.5 0.995 Ambrogi et al., 2008 MgAl-LDH−diclofenac Direct anion exchange Higuchi 0.5 0.989 Ambrogi et al., 2002 ZnAl-LDH/diclofenac /
Polycaprolactone Direct anion exchange Ritger-Peppas 0.37 0.933 Wang et al., 2019 ZnAl-LDH−diclofenac Direct anion exchange Ritger-Peppas 0.21 0.991 Joy et al., 2017 ZnAl-LDH−diclofenac Direct anion exchange Higuchi 0.5 0.986 Perioli et al., 2011
† This publication is dedicated to the memory of our mentor, friend and colleague, Prof. István Pálinkó, who passed away shortly after the submission of the current manuscript
*corresponding author
sipos@chem.u-szeged.hu (Pál Sipos)
2
5 10 15 20 25 30 35 40 45 50 55 60
2 theta /
dehydroxylation-rehydration
Intensity /a.u. (32 nm)
87.5% v/v ethanol 12.5% v/v water 75% v/v ethanol 25% v/v water 50% v/v ethanol 50% v/v water 25% v/v ethanol 75% v/v water
400C heat treated CaFe-LDH
LDH Ca2Fe2O5
(35 nm)
(18 nm)
(8 nm)
CaCO3
Fig. S1 X-ray diffractometry patterns of diclofenac anion-intercalated LDH samples intercalated in ethanol-water mixtures of varying compositions (1:1 Fe(III):diclofenac anion molar ratio, 25°C) and the as-prepared LDH after calcination at 400°C.
5 10 15 20 25 30 35 40 45 50 55 60
2 theta /
dehydroxylation-rehydration
LDH
Intensity /a.u.
75C
50C
25C (35 nm)
CaCO3
Fig. S2 X-ray diffraction patterns of solids obtained at varying stirring temperature (1:1 Fe(III):diclofenac anion molar ratio, 25% v/v ethanol-water mixture).
3
5 10 15 20 25 30 35 40 45 50 55 60
2 theta /
dehydroxylation-rehydration
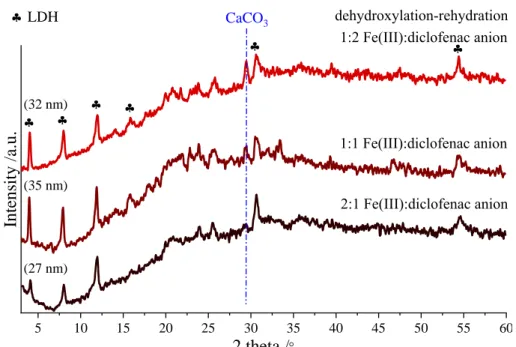
(35 nm)
(27 nm) (32 nm)
LDH
Intensity /a.u.
1:2 Fe(III):diclofenac anion
1:1 Fe(III):diclofenac anion
2:1 Fe(III):diclofenac anion
CaCO3
Fig. S3 X-ray diffraction patterns of the LDH composites at different Fe(III):diclofenac anion molar ratios (25% v/v ethanol-water mixture, 25°C).
4
Table S2
Crystal, size, heterogeneity parameters and zeta potential of pristine and organic CaFe-LDH solids (average predominant solvodynamic diameter – Zavg., polydispersity index – PDI, average zeta potential – avg.).
Samples d-value
(nm)x a
(nm)y Zavg. (nm) PDI avg. (mV)
CaFe−NO3-LDH 0.854 0.586 255 ± 60 0.42 −12.9 ± 1.6
co-precipitation LDH−diclofenac 2.291 0.586 1220 ± 330 0.13 −9.5 ± 1.1 direct anion exchange LDH−diclofenac 2.304 0.582 1140 ± 330 0.17 −9.3 ± 2.7 dehydroxylation-rehydration LDH−diclofenac 2.271 0.586 675 ± 185 0.35 −11.5 ± 3.4 mechanochemically-aided intercalation LDH−diclofenac 2.276 0.584 540 ± 170 0.21 −12.4 ± 1.8 co-precipitation LDH−naproxen 1.943 0.586 760 ± 180 0.46 −4.7 ± 1.5 direct anion exchange LDH−naproxen 1.935 0.586 2280 ± 605 0.22 −5.4 ± 3.4 dehydroxylation-rehydration LDH−naproxen 1.926 0.584 2770 ± 760 0.22 −2.5 ± 1.3 mechanochemically-aided intercalation LDH−naproxen 1.902 0.584 3125 ± 585 0.45 −3.9 ± 1.4
x d-value = d001 or the first reflections
y Lattice parameter a is calculated by estimation: a = 2d110
5
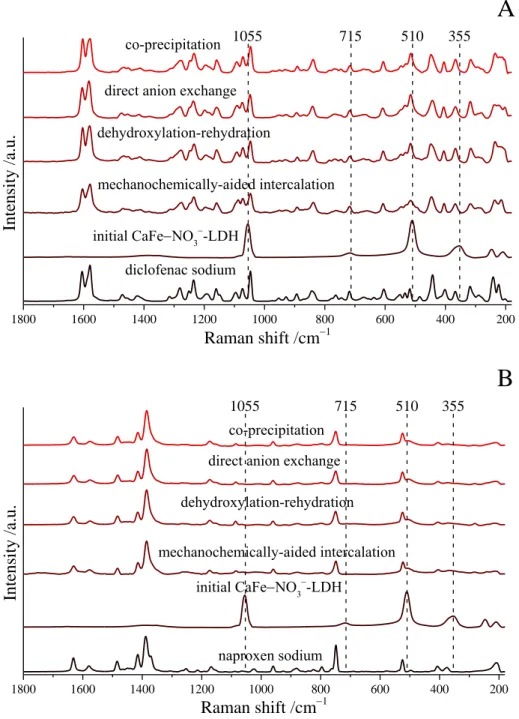
1800 1600 1400 1200 1000 800 600 400 200
A
Intensity /a.u.
Raman shift /cm1 co-precipitation
direct anion exchange dehydroxylation-rehydration
mechanochemically-aided intercalation initial CaFeNO3-LDH
diclofenac sodium
510
1055 715 355
1800 1600 1400 1200 1000 800 600 400 200
B
Raman shift /cm1
510 1055
Intensity /a.u.
co-precipitation direct anion exchange dehydroxylation-rehydration mechanochemically-aided intercalation
initial CaFeNO3-LDH
naproxen sodium
715 355
Fig. S4 Raman spectra of the diclofenac (A) and naproxen (B) anion-intercalated LDH samples and those of the as-prepared CaFe-LDH and starting drugs.
6
5 10 15 20 25 30 35 40 45 50 55 60
20.69
29.54
19.84
27.02
22.46
27.8
26.98
23.43
17.12
15.12
8.48
6.02
17.52
13.07
4.42
Intensity /a.u.
2 theta /
diclofenac sodium
naproxen sodium
Fig. S5 X-ray diffractograms of the naproxen and diclofenac sodium salts.
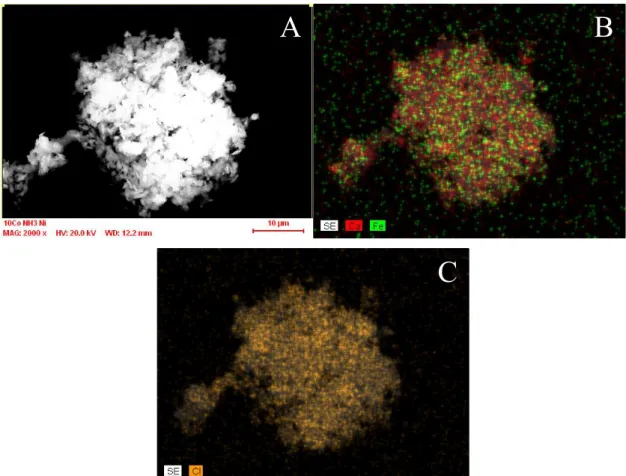
Fig. S6 Energy dispersive X-ray analysis spectrum of diclofenac anion−CaFe-LDH composite (signals of silicon and aluminium are originated from the adhesive tape/sample holder).
7
Fig. S7 SEM (A) and elemental map (B and C) images from the diclofenac anion−CaFe-LDH.
A B
C
8
100 200 300 400 500 600 700 800 900
60 65 70 75 80 85 90 95 100
Mass /%
Furnace temperature /C
Deriv. Mass /%/min
CaFe-NO3 LDH
-0.7 -0.6 -0.5 -0.4 -0.3 -0.2 -0.1 0.0 0.1
690
245 125
515
100 200 300 400 500 600 700 800 900 1000
0 20 40 60 80 100
Furnace temperature /C
-0.4 -0.3 -0.2 -0.1 0.0
Mass /%
620 515 diclofenac sodium
285
315 870
Deriv. Mass /%/min
100 200 300 400 500 600 700 800 900 1000
0 20 40 60 80 100
Furnace temperature /C
750
800
Mass /%
naproxen sodium
75
380 450
720
Deriv. Mass /%/min
-0.6 -0.5 -0.4 -0.3 -0.2 -0.1 0.0
Fig. S8 Thermal behaviour of the pristine CaFe-LDH and sodium salts of diclofenac and naproxen.
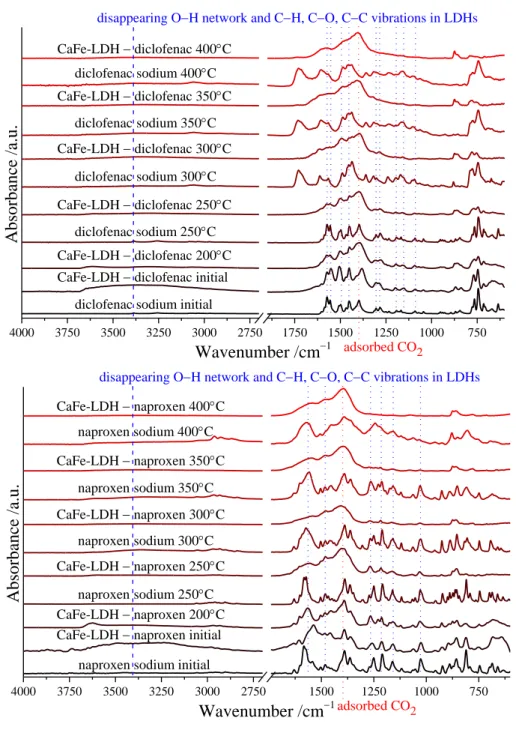
9 4000 3750 3500 3250 3000 2750 1750 1500 1250 1000 750
adsorbed CO2
Wavenumber /cm1
Absorbance /a.u.
CaFe-LDH diclofenac 400C diclofenac sodium 400C CaFe-LDH diclofenac 350C
diclofenac sodium 350C CaFe-LDH diclofenac 300C
diclofenac sodium 300C CaFe-LDH diclofenac 250C
diclofenac sodium 250C CaFe-LDH diclofenac 200C CaFe-LDH diclofenac initial
diclofenac sodium initial
disappearing OH network and CH, CO, CC vibrations in LDHs
4000 3750 3500 3250 3000 2750 1500 1250 1000 750
disappearing OH network and CH, CO, CC vibrations in LDHs
CaFe-LDH naproxen 350C
CaFe-LDH naproxen 300C
CaFe-LDH naproxen 250C
CaFe-LDH naproxen 200C CaFe-LDH naproxen initial
naproxen sodium initial naproxen sodium 350C
naproxen sodium 300C
naproxen sodium 250C CaFe-LDH naproxen 400C
naproxen sodium 400C
Absorbance /a.u.
Wavenumber /cm1adsorbed CO2
Fig. S9 Infrared spectra of diclofenac, naproxen sodium salts and drug-intercalated (by the dehydroxylation-rehydration technique) LDH solids after heat treatments at various temperatures.
10
100 1000
0 5 10 15 20 25
Diameter (nm)
intercalated diclofenac anion
CaFe-NO3-LDH co-precipitation direct anion exchange dehydroxylation-rehydration mechanochemically-aided intercalation
Number (%)
1150 1060 620 260 510
1000 10000
0 5 10 15 20 25
5000
Number (%)
Diameter (nm)
co-precipitation direct anion exchange
dehydroxylation-rehydration mechanochemically-aided intercalation 710
3240
2700 2200 intercalated
naproxen anion
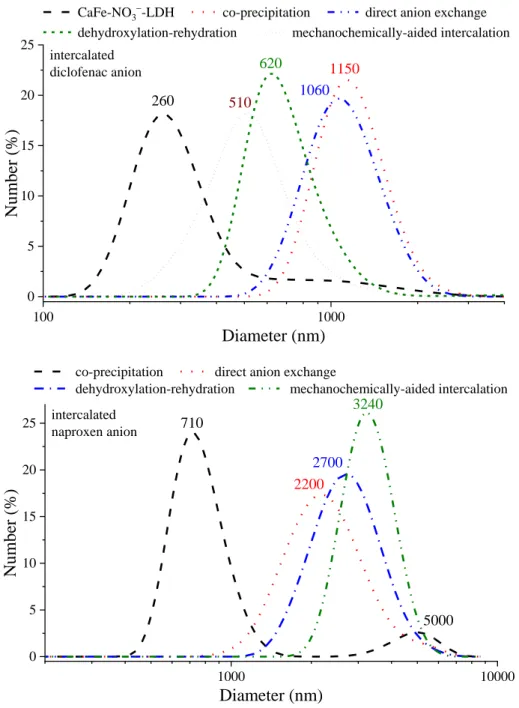
Fig. S10 The number-weighed size distribution patterns of the as-prepared (nitrate-containing), the diclofenac and the naproxen anion-intercalated CaFe-LDH particles (the numbers show the predominant solvodynamic diameters).
11
0 100 200 300 400 500 600 700 800 900 1000
1 5 10 25 50 75 100 200 400 600 800 0.1%
0.4% 0.1%
0.9% 1.5%
1.8%
6.2%
20.7%
47.9%
20.1%
co-precipitation naproxenLDH
Area /mm2
Number of particles
0 500 1000 1500 2000 2500 3000 3500 4000 4500 5000
1 5 10 25 50 75 100 200 400 600 800 0.04%
0.02%
1.1% 0.4%
2.4% 0.9%
7.7%
28.8%
43.4%
15.4%
direct anion exchange naproxenLDH
Area /mm2
Number of particles
0 100 200 300 400 500 600 700 800 900 1000
1 5 10 25 50 75 100 200 400 600 800 0.2% 0%
2.6% 1.4%
1.2% 1.5%
4.9%
14.9%
47.4%
26.0%
dehydroxylation-rehydration naproxenLDH
Area /mm2
Number of particles
0 100 200 300 400 500 600 700 800 900 1000
1 5 10 25 50 75 100 200 400 600 800 0.5% 0.2%
2% 1.1%
2.1% 1.5%
9.7%
24.1%
43.9%
14.5%
mechanochemically-aided intercalation naproxenLDH
Area /mm2
Number of particles
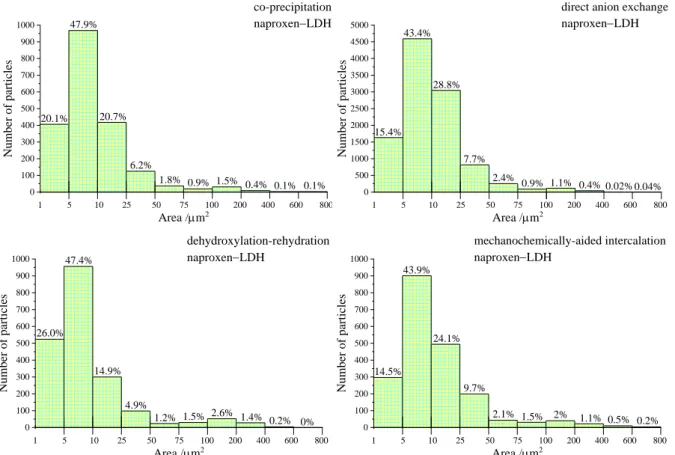
Fig. S11 Particle size distribution histograms of the naproxen anion-intercalated CaFe-LDH solids dispersed in hydrogels.
Ambrogi, V., Fardella, G., Grandolini, G., Perioli, L., Tiralti, M.C., 2002. Intercalation compounds of hydrotalcite-like anionic clays with anti-inflammatory agents, II: Uptake of diclofenac for a controlled release formulation. AAPS PharmSciTech. 3, 26.
https://doi.org/10.1208/pt030326
Ambrogi, V., Perioli, L., Ricci, M., Pulcini, L., Nocchetti, M., Giovagnoli, S., Rossi, C., 2008.
Eudragit and hydrotalcite-like anionic clay composite system for diclofenac colonic
delivery. Micropor. Mesopor. Mat. 115, 405–415.
https://doi.org/10.1016/j.micromeso.2008.02.014
Carriazo, D., del Arco, M., Martín, C., Ramos, C., Rives, V., 2010. Influence of the inorganic matrix nature on the sustained release of naproxen. Micropor. Mesopor. Mat. 130, 229–238.
https://doi.org/10.1016/j.micromeso.2009.11.014
Joy, M., Iyengar, S.J., Chakraborty, J., Ghosh, S., 2017. Layered double hydroxide using hydrothermal treatment: morphology evolution, intercalation and release kinetics of diclofenac sodium. Front. Mater. Sci. 11, 395–408. https://doi.org/10.1007/s11706-017- 0400-1
Perioli, L., Posati, T., Nocchetti, M., Bellezza, F., Costantino, U., Cipiciani, A., 2011.
Intercalation and release of antiinflammatory drug diclofenac into nanosized ZnAl hydrotalcite-like compound. Appl. Clay Sci. 53, 374–378.
https://doi.org/10.1016/j.clay.2010.06.028
Rojas, R, Jimenez-Kairuz, A.F., Manzo, R.H., Giacomelli, C.E., 2014. Release kinetics from LDH-drug hybrids: Effect of layers stacking and drug solubility and polarity. Colloid.
Surface. A 463, 37–43. https://doi.org/10.1016/j.colsurfa.2014.09.031
12
Wang, H., Wu, J., Zheng, L., Cheng, X., 2019. Preparation and properties of ZnAl layered double hydroxide/polycaprolactone nanocomposites for use in drug delivery, Polym-Plast.
Technol. 58,1027–1035. https://doi.org/10.1080/03602559.2018.1493121