My special thanks go to the members of the Department of Radiation Chemistry with whom I worked: Gergely Rácz, Dr. I would also like to express my sincere appreciation to the Article Reviewers for their work and kind criticism.
LITERATURE REVIEW
Free radicals in biology
- Oxidation of proteins, lipids and DNA
- Tyrosine oxidation
- Cysteine oxidation
- Methionine oxidation
- Oxidation of lipids and DNA
- Antibiotics as exogenous stressors of oxidative stress
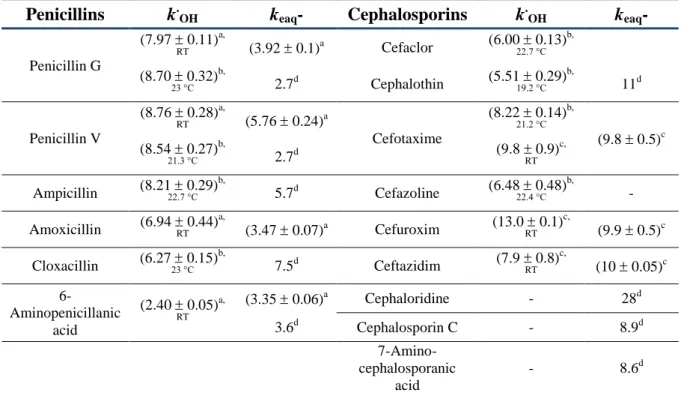
Due to the low dissociation energy of the RS-H bond (BDE = 367 kJ mol-1; R denotes the residual cysteine moiety in this section), thiols can easily donate a hydrogen atom to free radicals [11]. A key role is attributed to the oxidation of the nucleotide pool during antibiotic-mediated oxidative stress [65].
Introducing a special group of antibiotics: penicillins
The β-lactam ring is a reactive acylating agent that binds covalently to the serine residue of the enzyme and forms a practically dead acyl-enzyme complex. It follows that in the absence of the four-membered lactam ring (ie ring opening) they lose their antibacterial activity.
Generation of biologically relevant free radicals in vitro
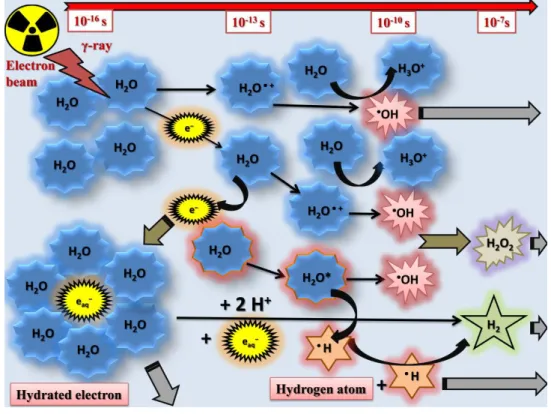
- Principles of radiation chemistry: water radiolysis
The number of photons (I expressed in [photons cm-2]) at a given depth (x in [cm]) of the absorber is given by: I = I0 × e-μx, where I0 is the intensity of the beam before penetration and μ is the linear absorption coefficient (in [cm-1]) of the medium of interest. High-energy electrons deposit their energy by exciting and ionizing the molecules of the medium.
Free radicals for environmental protection
- Antibiotic resistance
- β-Lactams as hazardous water pollutants
- Advanced oxidation of β-lactams for eliminating
In the case of cephalexin, the retention antibacterial activity was observed as a result of ferrate (VI) oxidation due to the significant antibacterial activity of the sulfoxide and sulfone end product [140]. Due to the intact remaining pharmacophore, the inability of OH to remove antimicrobial potency.
AIMS AND OBJECTIVES OF THE THESIS
Eaq− and OH cause the destruction of the β-lactam system, which determines the antibacterial activity. Nevertheless, the general mechanism of the OH-induced one-electron oxidation of penicillins, which forms the basis of several AOPs, has not yet been elucidated.
MATERIALS AND METHODS
Materials
Methods
- Irradiation
- Steady-state γ-irradiation
- Pulse radiolysis experiments
- Specific conditions to observe distinct free radical reactions
- Analytical techniques
- Spectroscopy
- Chromatography
- CO 2 release from the samples
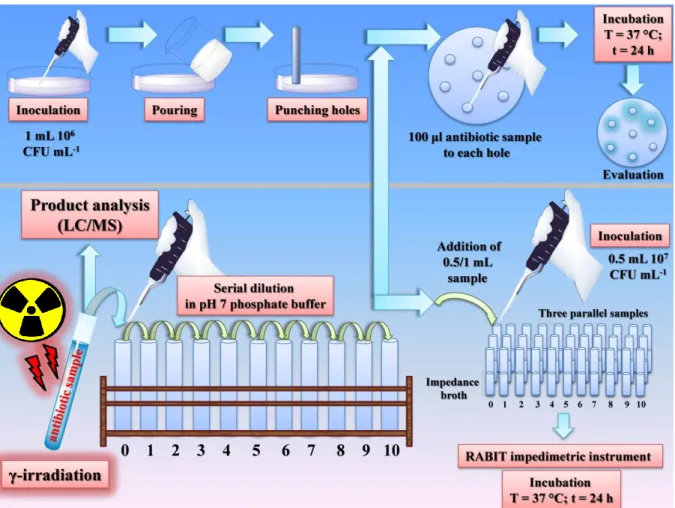
- Microbiological assays
- Toxicity tests: acute and chronic effects
- Bacterial susceptibility tests
The chemical radiation yield of CO2 release can be obtained from the slope of the yield ((∆TOC)/12 = (TOC0- TOCirradiated)/12) - dose plot. Schematic representation of the agar diffusion practice (upper part) and liquid macrodilution assay.
RESULTS AND DISCUSSION
Free radical induced oxidation mechanism of a penicillin derivative
- Primary steps of the OH induced oxidation
- Products forming under steady-state gamma irradiation
- Modifications of the amoxicillin structure
- The course of reactions leading to the S-oxide
- Concluding remarks
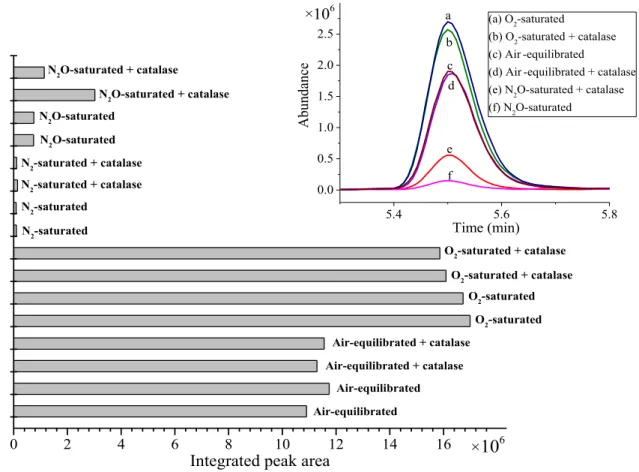
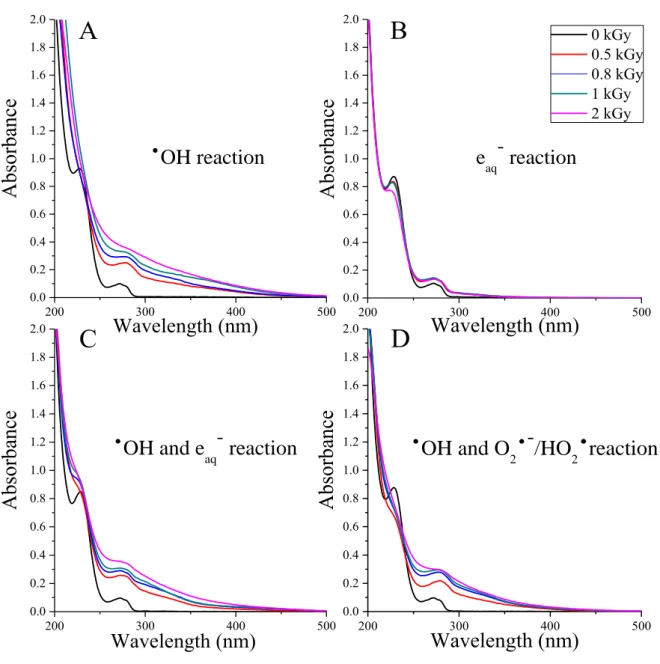
In fact, OH can attack the sulfur atom from different sides of the thiazolidine ring. Under these conditions, moderate yields of the sulfoxide were obtained (Figure 6), which could be attributed to the effect of either of these species [21,197]. In the absence of the >S.˙.S< dimer (vide supra), since the OH adduct at the sulfur has a long lifetime, it can readily react with oxygen forming the corresponding peroxyl radical (p).
This phenomenon cannot be attributed solely to the reduced yield of the potential precursors, OH and H2O2. However, it is attributed to the annihilation of the primary intermediate cation of the sulfur radical in the reaction with eaq. Therefore, it is proposed that the sulfur radical cation is the precursor of the sulfoxide under anaerobic conditions.
From the decrease in the relative yield of S-oxide (Figure 6) and the yield of H2O2, it can be estimated that in the system saturated with N2O ~ 60% of the sulfoxide yield can be attributed to the presence of H2O2. H2O2 reacts with organic sulfides at a lower rate compared to the reaction of the radical intermediates, e.g.
One-electron reduction mechanism of penicillin derivatives (Objective II)
- Kinetics of e aq
- The one-electron reduction mechanism
- Hydrated electron attack at the carboxylate group
- Hydrated electron attack at the β-lactam carbonyl
- Formation of benzyl radicals in ampicillin and amoxicillin samples
- Hydrated electron attack at the amide carbonyl
- Concluding remarks
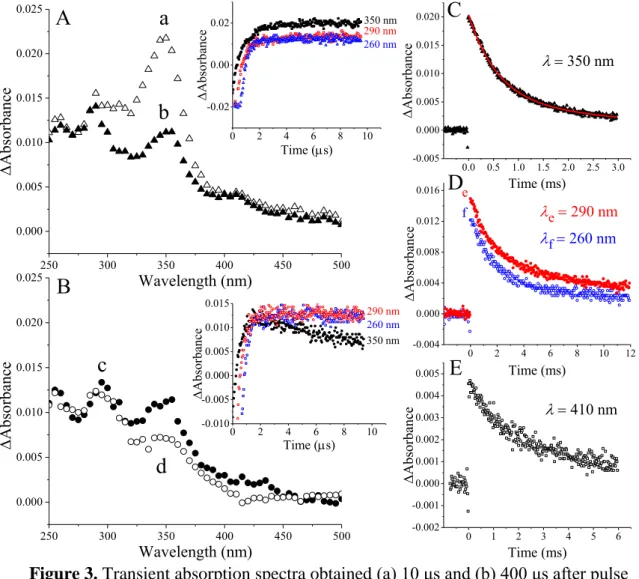
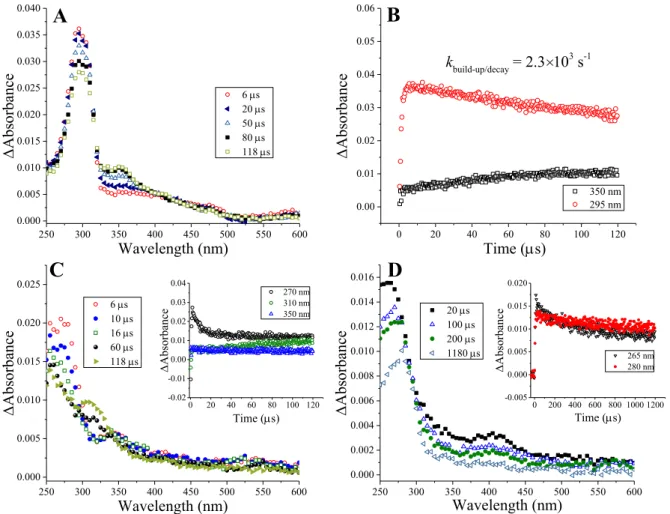
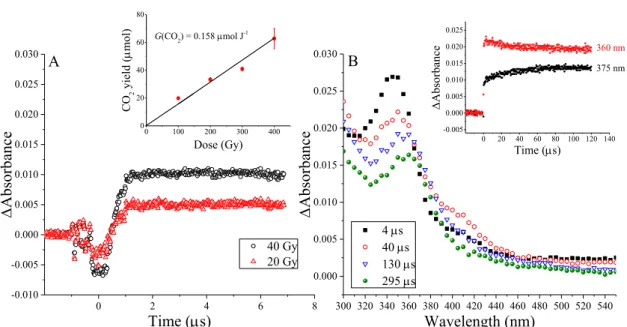
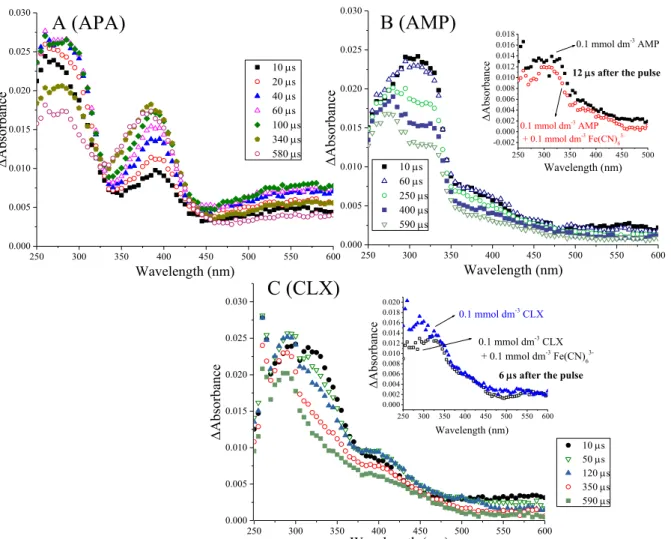
The carboxylate group of amoxicillin can be considered a C-terminal residue due to the similarity of its skeleton to a tripeptide. One-electron reduction of the β-lactam carbonyl group causes the formation of the corresponding ketyl radical anion (d) (Scheme 8). The transient spectra of the compounds of interest (amoxicillin, ampicillin, cloxacillin and 6-aminopenicillanic acid, Figure 8 and Figure 9) show that the first absorption band belongs to the corresponding α-hydroxyalkyl radical (e).
59 (Figure 8A, the inset of Figure 8B shows the kinetic trace at 380 nm), indicating absorption of the nascent intermediate. This effect is believed to occur due to the "twisted" (compact) conformation of penicillin derivatives [206, 207]. In the one-electron reduction of penicillin derivatives, intermediates of ketyl radicals are formed as a result of the placement of a hydrated electron on carbonyl carbons.
A special feature of the system is that the adduct to the carboxylate group is stable for several μs, similar to benzoic acid. The feasibility of the process can be revealed by determining the reduction potentials of one electron.
The e aq −
- Evidence that OH and e aq target the bicyclic system
- β-Lactam degradation initiated by OH
- β-Lactam degradation initiated by hydrated electron
- Kinetics of the penicillins + OH reaction
- Concluding remarks
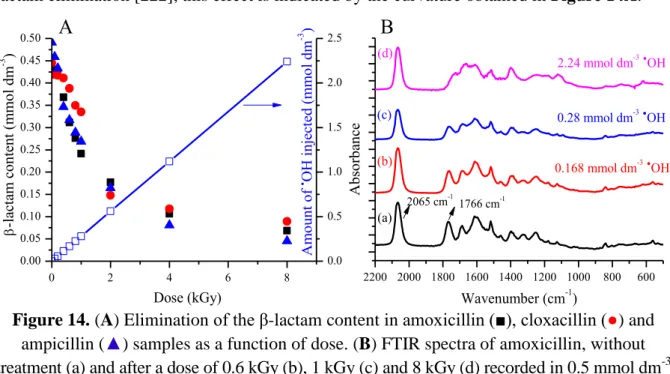
Here the destruction mechanism of the β-lactam system will be investigated in amoxicillin (AMX) as a model compound (Section 3, Graph 5). The radiation chemical yield of elimination of β-lactam content was found to be From the UV-Vis/IR results it appears that O2 slightly inhibits the elimination of the β-lactam ring.
The kinetics of the OH + penicillin reaction is discussed, which brings the amoxicillin molecule into focus. Since OH attacks various parts of the molecule (Section 5.1.2), the differential equation describing the decay of OH can be given as (I) indicates. This enables us to investigate the overall reactivity of the OH with a molecule of interest.
The deviation of the measured endpoint from linearity (Figure 12B) can be rationalized by considering the reaction of (SCN)2·. OH attack on sulfur yields intermediates that promote the destruction of the β-lactam system.
The effect of oxidation products of penicillin derivatives
- Chemical approach
- Following the fate of the pharmacophore that determines
- Investigating the structures of the products
- Biological approach
- Acute and chronic toxicity tests
- Bacterial susceptibility test: agar diffusion assay
- Bacterial susceptibility test: broth macrodilution assay
- Concluding remarks
To examine the mechanism of formation of the compounds in Scheme 9, we refer to the example of amoxicillin (Section 5.1). Some cloxacillin products exposed to a dose of 0.8 kGy in an air-equilibrated state. The formation of the (R) isomer is favored due to the H-bond with the amide group and due to steric hindrance in the case of the (S) isomer (with the carboxylate group) [228].
These isomers have a reduced antibacterial activity, which is less pronounced in the case of the (R) isomer in which the oxygen is located on the opposite side of the thiazolidine ring relative to the β-lactam ring [119]. These considerations can also be taken into account in the case of the products of amoxicillin oxidation (Section 5.1.2, Scheme 4). In summary, peak observed at low radical exposure is attributed to the increase in the permeability of the investigated penicillins.
This effect is due to the change in the hydrophilicity of the products compared to the parent compound (vide supra). When the logarithm of the residual antibiotic concentration (determined by LC/MS after the treatment) is plotted against the area under the growth curve, which represents the size of the bacterial population, a dose-response relationship is obtained (Figure 19A,B,E).
One-electron oxidation mechanism of penicillins in relation to
- One-electron oxidation of penicillins induced by OH
- One-electron oxidation of penicillins induced by Cl 2 −
- Reaction kinetics of Cl 2 −
- One-electron oxidation of penicillins induced by Cl 2 −
- One-electron oxidation of penicillins induced by Br 2 −
- Concluding remarks
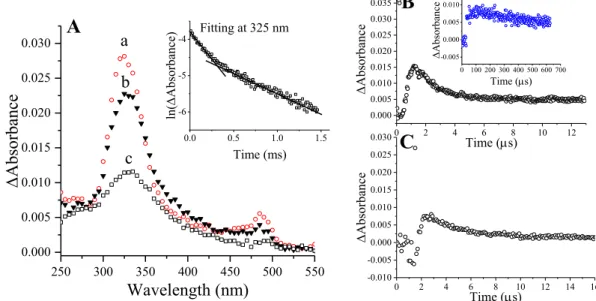
In order to follow the oxidation of the thioether moiety, 6-aminopenicillanic acid (APA) was chosen as the common substructural compound of penicillins. Due to the lack of an absorption band at λmax = 285 nm in the oxidation of 6-aminopenicillanic acid caused by Cl2·· (Fig. 22A, see below) and taking into account the similarity of the spectra in the oxidation caused by OH in the case of ampicillin (AMP) (Fig. 20 A, B), the band has a peak at 285 nm (Fig. 20 A) belongs to type a. Similar to the case of amoxicillin (Section 5.1.1), an adduct is expected in which the attack of the OH from the opposite side of the thiazolidine ring relative to the carboxylate group (in the absence of stabilizing effects), rapid elimination of the –OH, resulting in the formation of a sulfur radical cation (b).
It appears that this pathway is highly favored since, in the case of amoxicillin, ~30% of the initially available OH contributes to the formation of species f. It is clear from the determined rate constants that the sulfur atom is expected to be the predominant site for Cl2 −. This band rapidly disappears as the reaction proceeds, leading to the transient spectra of the intermediates formed.
This is easily understood if one considers the sulfur-stabilized adduct in the case of oxidation caused by OH (Figure 20A, λmax = 285 nm). The kinetics as well as the mechanism of the Cl2·/Br·2··induced oxidation show that sulfur is the predominant oxidation site.
SUMMARY
Ketyl radicals are reported to form in the case of redox-cyclic quinones, which underlies their toxicity. If the ketyl radicals of penicillins exhibit similar redox-cycling properties, it would provide an explanation for the oxidative stress phenomenon that is often concerned in the literature. Furthermore, it is expected that electron migration occurs from the ketyl radical of the carboxylate part towards the β-lactam nitrogen, which leads to opening of the β-lactam ring.
The relatively high efficiency provided by eaq and OH in eliminating the main β-lactam pharmacophore that determines the antibacterial activity is promising for the implementation of an advanced oxidation process to eliminate the residual antibacterial activity of wastewater matrices derived from penicillins. Excessive or insufficient radical exposure was found to be detrimental in eliminating the selective pressure on bacterial strains. This may be due to the products' improved permeability to both gram-negative and gram-positive species.
The negative effect of high radical exposure presumably arises from the formation of polyhydroxylated phenolic compounds. A low radical exposure may occur under real treatment conditions, since most of the OH is removed by the complex matrix of the wastewater (natural alkalinity, dissolved organic matter).
NOVEL SCIENTIFIC FINDINGS
LIST OF PUBLICATIONS
Analytical approach to OH radical-induced degradation of sulfonamide antibiotics in dilute aqueous solutions. Report of the 2nd Research Coordination Meeting (RCM) on "Irradiation Treatment of Wastewater for Reuse with Special Focus on Wastewater Containing Organic Pollutants" October 29 - November 2, 2012, Jeongeup, Republic of Korea, 67-78. http://www-naweb.iaea.org/napc/iachem/working_materials/RC-1188-2-report.pdf). OH-Induced Oxidation of Penicillins in Relation to Advanced Oxidation Techniques: Elimination of Antibacterial Activity.
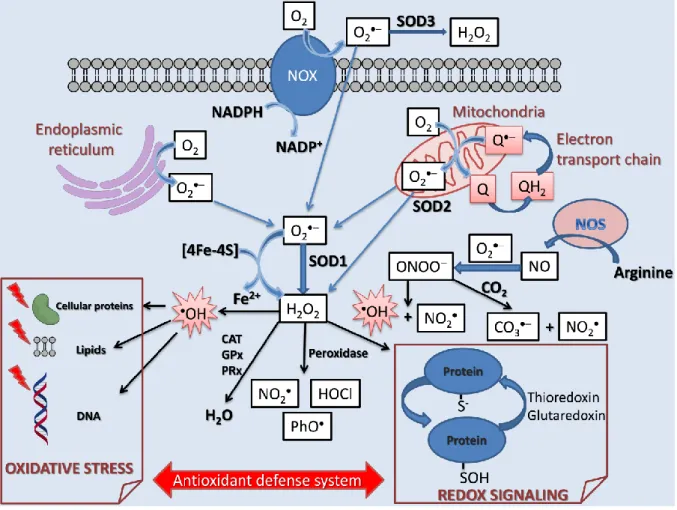
Among the seven identified sites of mitochondrial superoxide formation, complex I (NADH dehydrogenase) and complex III (ubiquinone-cytochrome c reductase) in the electron transport chain have the greatest capacity [4]. Several measures ensure the safe operation of the irradiator, which can be operated from a separate room. This equipment is connected to the upper part of the tube via a Freon-filled waveguide.
The electromagnetic deflection system is located near the exit window of the electron beam. In addition, the absorbed dose is also a function of vacuum and magnetron current. Optical detection is based on the absorption of the analyzing light by the emerging transition species.
Furthermore, the I0 level is also conserved as it is necessary for absorption extraction (see below). Due to the loss of the carboxyl group, Rt shifted to a longer time compared to amoxicillin (from 6.279 to 8.099 min). The carbonyl stretch of the carboxyl group and the amide N-H bend are assigned to the absorption band peaking at 1685 cm-1.
The fragments at m/z 90 and m/z 75 are attributed to the decomposition of the benzene ring after HCl loss.