Symposia Biologica Hungariea
2 4NEW
APPROACHES IN LIQUID
CHROMATOGRAPHY
Akadémiai Kiadó, Budapest
NEW APPROACHES IN LIQUID CHROMATOGRAPHY
E d ite d by H . K ALÁSZ
(Symposia Biologica Hungarica 24)
The volum e co n tain s selected papers deliv
ered a t th e 2nd A nnual A m erican -E astern E uropean Sym posium on A dvances in Liquid C hrom atography held in Szeged, H ungary, Ju n e 16-18, 1982.
The m ost recen t resu lts in displacem ent chro
m a to g rap h y using m a th em atical modelling of separation techniques arc presented. D is
placem ent m ode o f developm ent on thin- layer p la te s an d th e effect of th e length of developm ent, sam ple size an d position of spots in th e se p aratio n of stru c tu ra lly related com pounds are also elab o rated .
Some p ap e rs deal w ith th e characterization of th e s ta tio n a ry p h ase u nder real chrom a
tographic co nditions as well as th e possibility of optim ization of H P L C and gel chrom a
tography.
New results in TLC fo r d eterm in atio n of b e
ta-blocking ag en ts from h um an urine and for th e relatio n s of th e ch rom atographic b eh a v iour an d chem ical s tru c tu re of nitrogen- bridged organic com pounds are also given.
A m erican, Czechoslovak, G erm an , H u n g aria n , Polish an d Soviet scientists have provided a cross-section of new theoretical aspects and p ractical applicatio n of liquid chrom a
tography.
AKADÉMIAI KIADÓ BUDAPEST
IS B N 963 05 3555 6
Symposia
Biologica
Hungarica
24
Symposia Biologica Hungarica
Yol. 24
R e d ig it H . KALÁ SZ
AKADÉMIAI KIADÓ, BUDAPEST 1984
NEW APPROACHES IN LIQUID
CHROMATOGRAPHY
Proceedings of the 2nd Annual American-Eastern European Symposium
on Advances in Liquid Chromatography, Szeged, Hungary, June 16—18, 1982
E d ite d by
H. KALÁSZ
D ep a rtm e n t o f P harm acology Semmelweis U n iv ersity o f M edicine
B u d ap est, H u n g ary
AKADÉMIAI KIADÓ, BUDAPEST 1984
T his volum e is published by E lsevier Science P ublishers as Vol. 16 in th e A nalytical C hem istry Sym posia Series
J o in t edition p ublished by E lsevier Science P ublishers, A m sterdam , The N eth erlan d s a n d A kadém iai K iad ó , The P ublishing H ouse o f th e H un g arian A cadem y o f Sciences,
B udapest, H u n g a ry
IS B N 963 05 3556 6
© A kadém iai K iadó, B u d a p est 1984
P rin te d in Hunwary
FOREWORD
I n c h r o m a t o g r a p h i c w o r k s c l a s s i c a l d e f i n i t i o n s c an be r e a d . One o f t h e b a s i c b o o k s d e c l a r e s f o r i n s t a n c e t h a t " c h r o m a t o g r a p h y o p e r a t e s a s a s y s t e m o f t h r e e c o m p o n e n t s . A s t a t i o n a r y p h a s e , a m o b i l e p h a s e a n d , l a s t b u t n o t l e a s t , t h e s a m p l e w h i c h i s s u p p o s e d t o c a r r y t h r o u g h o r a l o n g t h e s t a t i o n a r y p h a s e . "
I b e l i e v e t h a t c h r o m a t o g r a p h y o p e r a t e s as a s y s t e m o f f o u r c o m p o n e n t s , and t h e f o u r t h one i s t h e p e r s o n a l c o n t a c t b e t w e e n
p e o p l e who do t h e f o b i n p a r t i c u l a r f i e l d s o f c h r o m a t o g r a p h y . A w o r k s h o p o r s y m p o s i u m m i g h t be a s i m p o r t a n t a s t h e wor k w h i c h i s ( o r s h o u l d b e ) p r e s e n t e d . The p e r s o n a l c o n t a c t and e x c h a n g e o f t h o u g h t s have a m o s t p o w e r f u l c a t a l y t i c a c t i o n on s c i e n t i f i c w o r k . The i n s p i r a t i o n g a i n e d f r o m o t h e r s may h a v e a b a s i c r o l e i n t h e d e v e l o p m e n t o f a new t e c h n i q u e , b u t e s p e c i a l l y i n t h e d i s s e m i n a t i o n o f a new m e t h o d . C h r o m a t o g r a p h y i s an e x t r e m e l y r a p i d l y - g r o w i n g f i e l d o f m e t h o d o l o g y . The l a r g e amount o f new i n s t r u m e n t s , c h e m i c a l s , s o l i d p h a s e s , t r i c k s , t o o l s a n d i d e a s f o r m a p u z z l i n g p i c t u r e w h i c h may be c l a r i f i e d by t a l k i n g and e x c h a n g i n g i d e a s w i t h p e o p l e who h ave a l r e a d y had some p e r s o n a l e x p e r i e n c e w i t h a g i v e n s u b j e c t , o r t e c h n i q u e .
A S ym p o s i u m d e a l i n g w i t h "New A p p r o a c h e s i n C h r o m a t o g r a p h y "
c o u l d be j u s t a n o t h e r s y m p o s i u m , wer e i t n o t f o r a v e r y s p e c i a l c h a r a c t e r i s t i c . And t h i s S y m p o s i u m had o n e , n a m e l y t h e v e r y t i g h t c o n n e c t i o n b e t w e e n E a s t a n d W e s t . I t was t h e s e c o n d Sym
p o s i u m i n t h i s s e n s e . S c i e n t i s t s c o m i n g f r o m E a s t a n d W e s t, t h e i r i n t e r e s t i n g p a p e r s a nd l i v e l y d i s c u s s i o n s d e m o n s t r a t e d t h e f r u i t f u l n e s s o f s u c h m e e t i n g s . T h i s c o l l e c t i o n o f p a p e r s
V
w i l l d e m o n s t r a t e t o a l l who d i d n o t p a r t i c i p a t e i n t h i s Sym
p o s i u m t h a t t h e r e w er e many i m p o r t a n t t o p i c s o f g e n e r a l i n t e r e s t f o r p e o p l e w o r k i n g i n t h i s f i e l d .
We t h a n k a l l t h o s e who p r e p a r e d t h e i r p a p e r s f o r p u b l i c a - t i o n j and we hope t o m e e t t h e p a r t i c i p a n t s o f t h e S y m p o s i u m a g a i n 3 a s w e l l a s many o t h e r s who w i l l d e c i d e } on r e a d i n g t h i s v o l u m e j t o p a r t i c i p a t e i n o u r n e x t m e e t i n g .
TIBOR DÉVÉNYI
VI
CONTENTS
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY
Separation of amino-substituted biologically active 3 derivatives of beta-melanotropin by reversed-phase
high-pressure liquid chromatography
VARGA J.M., AIROLDI L . , DAVILA-HUERTA G.
High-performance liquid chromatography of fibrous 13 proteins
DEYL Z ., MACEK K.
Adsorption chromatography of flexible polymer 23 molecules
GLÖCKNER G.
The evaluation of molecular connectivity indices and 35 Van der Waals volumes for correlation of chromatographic parameters
BOJARSKI J., EKIERT L.
DISPLACEMENT CHROMATOGRAPHY
Efficiency of separation processess as applied to 45 displacement chromatography
VERESS G.E., HORVÁTH CS., PUNGOR, E.
Effect of operating conditions in displacement thin- 57 layer chromatography
KALÁSZ, H., HORVÁTH C S .
VII
VIII
CHARACTERIZATION OF LIQUID CHROMATOGRAPHIC STATIONARY PHASES
"Chromsil". A new family of chromatographic packings 71 OHMACHT, R . , MATUS Z.
The role of the specific surface area of an adsorbent 85 in the optimization of mixture separation conditions
in thin-layer chromatography ROZYLO J.K., MALINOWSKA I.
OPTIMIZATION POSSIBILITIES IN LIQUID COLUMN CHROMATOGRAPHY
Critical evaluation of optimization methods for HPLC 103 VAJDA J., LEISZTNER L.
The mobile phase in liquid chromatography 109 ISSAQ H.J.
Optimization of gel chromatographic separations. 129 Numerical evaluation of gel chromatographic elution
curves, optimal sample size and fractionation KALÁSZ H., NAGY J., KERECSEN L.
THIN-LAYER CHROMATOGRAPHY
Determination of beta-blocking agents in human urine 159 by thin-layer and gas chromatography
PUCSOK J . , HOLLÓSI I.
Chemical structure and liquid chromatographic behaviour 165 among nitrogen-bridged compounds
SHALABY A., BUDVÁRI-BÁRÁNY ZS., HANKÓ-NOVÁK K., SZÁSZ GY., HERMECZ I.
IX ANALYSIS OF AMINO ACIDS
Screening for amino acid metabolism disorders by 191 ion-exchange thin-layer chromatography
KOVÁCS J., KISS P.
Correlation of phenylalanine and tyrosine values 201 determined by continuous flow fluorometry and amino
acid analyzer
MRSKOá A., POSPISIL R . , KOLCOVÁ V.
Oral loading test with L-methionine 205
POSPISIL R . , MRSKOS A., PODHRADSKÁ 0 - , STOURACOVÁ 0.
The metabolic changes of amino acids in maternal blood 209 and milk during pregnancy of healthy and phenylketonuric mothers
HYÁNEK J., VILETOVÁ H., TRNKA V., KUNOVÁ V., CERVENKA J.
ANALYTICAL AND PREPARATIVE SEPARATION OF PEPTIDES AND PROTEINS
Liquid chromatography, thin-layer chromatography and 217 high-performance liquid chromatography of oxytocin,
vasopressin, some of their specific analogues and fragments
BALÁSPIRI L. , TÓTH M.V., FEKETE T- , JANÁKY T., LÁSZLÓ F .A ., TÓTH G., SIR0KMÁN F.
Micro column chromatography of large peptides 231 GANKINA E.S., KOSTIUK 1,0., BELINKII B.G.
Separation of protein-deprenyl adducts by gel
chromatography 241
SZÖKŐ É ., KALÁSZ H., MAGYAR K.
X
Purification of superoxide dismutases from different 245 sources
MATKOVICS B., SZABŐ L.
Application of liquid chromatographic methods in the 261 biochemical analysis of tumor cell membranes
KREMMER T., TÓTH T., HOLCZINGER L.
Novel purification procedure of alpha-fetoprotein 275 MOLNÁR I.
List of Contributors 285
Index 289
H I G H -P E R F O R M A N C E L I Q U I D CHROMATOGRAPHY
P r o a , o f t h e Symp. on A d v a n c e s i n L i q u i d C h r o m a t o g r . , S z e g e d , H u n g a r y , 1982
S E P A R A T I O N OF A M I N O - S U B S T I T U T E D B I O L O G I C A L L Y A C T I V E D E R I V A T I V E S OF B E T A - M E L A N O T R O P I N BY R E V E R S E D - P H A S E
H I G H - P R E S S U R E L I Q U I D CHROMATOGRAPHY
J . M , VARGA, L. A I R O L D I , G, DAVI LA- HUERTA
Department of Dermatology, Yale University School of Medicine, New Haven, Connecticut 06510, USA
SUMMARY
1) Amino-substituted derivatives of ß-MSH were synthesized for v a r i
ous purposes such as studies on MSH receptors (FITC-MSH), for the produc
tio n of th io lated intermediates of ß-MSH (SPDP-MSH) and fo r studying the effects of a novel MSH-toxin conjugate (ouabain MSH).l) A mixture of FITC- -ß-MSH derivatives was separated by RPLC. The formation of FITC-MSH was monitored by simultaneous UV and fluorescence detection. The major fractio n of fluorochrcme labeled peptide was resolved as a homogeneous peak on re - chramatography. The tyrosinase stimulatory a c tiv ity of the conjugate vas 55” th a t of the unsubstituted peptide (10~8m) .
2) SPDP-MSH conjugates were separated by RP-HPLC by using a m ultiple a c e to n itrile gradient. Several biologically active SPDP-ßdMSH fractions were obtained and were used fo r the preparation of th iolated ß-MSH as in te r mediates to lin k the hormone to MSH-react ive biologically active molecules.
3) Ouabain-ß-MSH conjugates were separated by RP-HPLC from unconjugat
ed ß-MSH and from oxidized products of ouabain. The formation of reaction products was monitored by using (3h) -ouabain as tracer and measuring the rad io activ ity of the fractions. The tyrosinase-stim ulatory a c tiv ity of the major ouabain-MSH conjugate was 66% of th at of the unsubstituted peptide when measured a t 0.1 yM concentration. While free ouabain (1 yM) was highly cytotoxic to murine melancma, the ouabain-ß-MSH conjugate had a potent growth-stimilatory effect during the early phase of c e ll growth.
INTRODUCTION
Currently, High Pressure Liquid Chromatography (HPLC) is the method of choice for the separation and p u rificatio n of snail and mediun-sized (up to 20 residues) peptides and th e ir derivatives (1,2). Reversed-Phase HPLC
(RPLC) has been used successfully fo r the separation of biologically active N-hydroxyphenyl popionyl derivative of a ß-melanotropin (ß-MSH) fragment
(1,3). Subsequently, RPLC was used to monitor the time course of ß-MSH iodination and to purify the biologically active mono-^-35x-g_MSH derivative
(4). In addition to the production of biologically active radio-labeled MSH, we have been using RPLC for the p u rificatio n of biologically active amino-
substituted derivatives of the same as reported here.
3
The amino acid sequence of porcine ß-MSH ± s as shown:
Asp -Glu -Gly-Pro -Tyr -Lys -Met -Glu -His -Phe -Arg -Trp -Gly-Ser -Pro -Pro -Lys -Asp When the octadecapeptide is derivatized with amino-react ive reagents such as aldehydes, isothiocyanates or N-hydroxysuccinixnidylates, the forma
tio n of mixture is anticipated, consisting of three mono-substituted de
riv ativ es (in the N-terminai amino group or the e-anino groups of lysine
#6 and #17), three d isubstituted derivatives and one tri-su b s titu te d peptide.
In order to be able to use the conjugates fo r studies on MSH receptors they have to be separated frcm the unsubstituted peptide, from the reagents and fron the biologically inactive reaction products. As we show in th is report, RPLC is a convenient and rapid method for the p urificatio n of biologically active anino-substituted derivatives of ß-MSH.
METHODS 1) FITC-ß-MSH
To a solution of 4 mg HPLC-purif ied ß-MSH (4) in 1 ml 0.1M Na2(X>3, 0.7 mg fluorescein isothiocyanate (FITC, Signa, St. Louis, MO) in 1 ml 0.1M Na2<X) 2 was added and was allowed to react a t rocm tsnperature for 2 hr. The clear solution was f ilte r e d through a Biogel P2 column (0.8 x 20 cm
equilibrated with d is tille d w ater). The yellow fraction eluted in the void volume was freeze-dried, then redissolved in 0.4 ml d is tille d water.
Exposure to lig h t was reduced by wrapping the Biogel column and other containers of the reaction mixture in aluminium f o il . 10-250 y l aliquots of the solution were injected into the HPLC systan which consisted of two Waters Associates M6000A pumps, a Model 6600 Programmer, U6K in jecto r.
Waters yBondapack C18 0.3 x 30 cm column, Perkin-Elmer LC-75 UV/Vis de
te cto r, Perkin-Elmer fluorescence detector and Houston Instruments IXial Pen chart recorder. A lin ear 20-25% a c e to n itrile gradient was used in 5rM ammonium acetate (pH 5.8). Biological a c tiv ity of the fractions was meas
ured in Cloudnan S91 melanana c e lls by the Pcmerantz tyrosinase assay (5).
2) SPDP-p-MSH
To 200 yg HPLC-purified ß-MSH in 40 yl phosphate-buffered saline, ph 7.5 (PBS), a solution of 248 yg N-succinimidyl 3 - (2-pyridyl-dithio) propianate (SPDP, Pharmacia Fine Chemicals, Uppsala, Sweden) in 80 yl
4
I
ethanol was added and the reaction mixture was kept a t roan temperature for % hr. The unreacted reagent was neutralized by adding 488 yg ethanol- amine in 80 y 1 PBS. The reaction mixture was injected into a Beckman Model 334 Gradient Liquid Chranatograph which consisted of 2 Model 110 A pumps, a Model 420 microprocessor/progranmer, a Waters C18 yBondapak column a fixed wavelength UV detector and a Kipp & Zonen BD 41 two-pen recorder. An aceto
n i t r i l e gradient was used containing 0.05% triflu o ro a c e tic acid. The aceto
n i t r i l e concentration was increased according to the following schedule:
10 + 24% in 22 minutes, •+ 30% in 3 min, ■+ 40% in 30 min, -► 60% in 5 min.
0.5 ml fractions were collected, freeze-dried and assayed for tyrosinase a c tiv ity (5,6).
3) Ouabain-ß-MSH
The procedure we used was sim ilar to the one used for the preparation of dauncmycin-MSH (7). To a solution of 10 mg Ouabain (Signa) containing 5 yCi of ( H)-Ouabain (New England Nuclear, Boston, MA), 5 mg NalO^ was ■3
added in 0.5 ml PBS. The oxidation was allowed to proceed a t rocm tempera
ture for 1 hr. The reaction was terminated by adding glycerol to a 50 nM fin a l concentration. To the oxidized ouabain, 15 mg ß-MSH was added in 0.5 ml ^CO^ (0.15 M, pH 9.5). Followring 1 hr reaction at roan temperature, the
Schiff base was reduced by adding 0. 5 mg NaBH^ in 0.1 ml d is tille d water.
The reduction was completed a t 37° for 2 hr. The reaction mixture was passed through a Biogel P2 column (0.8 x 20 cm) equilibrated with d is tille d water. The radioactive fractio n appearing in the void volume was freeze- dried then redissolved and aliquots were injected into the Waters HPLC System described in Section 1. The system was operated in the iso cratic mode with 24% a c e to n itrile in 0.1 M (NH^PO^ pH 5.8). Radioactivity was measured in a Beckman LS 7000 Liquid S c in tilla tio n Counter.
RESULTS and DISCUSSION 1) FITC-ß-MSH
Fluorochrane-labeled ß-MSH has been used e a rlie r fo r c e ll surface labeling and for studies of hormone internalizatio n by melanoma c e lls (6).
In these e a rlie r studies, the FITC-peptide conjugates were separated frcm the reagents by gel exclusion chromatography. As is shown in Figure 1, the reaction mixture excluded frcm Bipgel P2 contains a small amount of un-
5
ABSORBANCEAT280 nm {•
Figure 1 Separation of FITC-a -MSH derivatives, 5 /AL FITC- ß -MSH solution was injected into the Waters HFLC System at room temperature. Flow ra te = 2 ml/min; fluorescence excitation a t 490 nm, emission measured a t 512 nm.
6
FLUORESCENCE(rel.units) (•
ABSORBANCE AT 280 nm (■
TIME (min)
a3
CD
UJo
LÜ
O
m
LÜcr
o
3_JU_
Figure 2 Re-chromatography of FITC- ß MSH fraction B (reference to Figure 1). See d e ta ils i s the Methods section. Conditions of HPLC were the
same as lis te d in Figure 1.
ABSORBANCEAT280nm
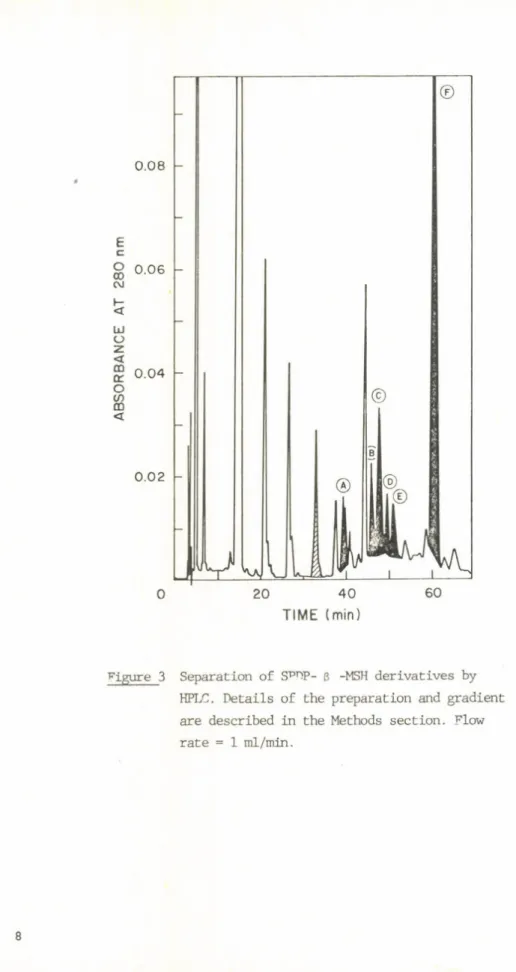
Figure 3 Separation of SPTTP- ß -MSH derivatives by HFLC. D etails of the preparation and gradient are described in the Methods section. Flow ra te = 1 ml/min.
labeled peptide. In addition, seven fluorochrcme labeled peptide fractions could be detected when the reaction mixture was separated by RP-HPLC. The biological a c tiv ity of the peaks was measured and compared to the
tyrosinase-stimmlatory a c tiv ity of unsubstituted ß-MSH (10-®M). The a c tiv it ie s of peaks A,B and C were 50, 57 and 2314 th a t of the unsubstituted peptide, respectively. The top fractions of peak B (eluted a t 19-20 min) were collected and freeze-dried. The re-dissolved FITC-ß-MSH was re-chrcma- tographed under iden tical conditions. As is shown in Figure 2, the seeming
ly homogeneous peak could be resolved on re-chromatography. This fraction was collected, freeze-dried and used for studies on MSH receptors. The biological a c tiv ity at 10 concentration was 55% that of the unsubstitu
ted hormone.
2) SPDP-ß-MSH
The N-succinimidyl-3- (2-pyridyldithio) -propionyl (SPDP) derivative of ß-MSH was prepared in order to introduce a th io l group into the peptide.
We used published methods of der ivat iz a t ion with SPDP (8). The separation of the reaction products is an example of the use of RPLC for purifying biologically active hormone derivatives from complex reaction mixtures
(Figure 3). In th is Figure the unshaded peaks show reaction products that were formed in the reaction of SPDP wriLth ß-ethanolamine alone ( i . e . , with the substance used to neutralize excess SPDP). The line-shaded peak shows the position of unsubstituted ß-MSH. The black peaks show the products formed in the reaction of ß-MSH and SPDP. When compared with the a c tiv ity of the unsubstituted peptide a t 10_^M, the tyrosinase-stim ulatory a c tiv ity of peaks A-E is in the 50-150% range; the a c tiv ity of peak F is uncertain.
Fractions were subsequently treated with d ith io th re ito l to reduce the d ith io bond and thus form th ila te d ß-MSH which was then used for producing MSH-taxin conjugates. D etails of th is work wri.ll be published la te r.
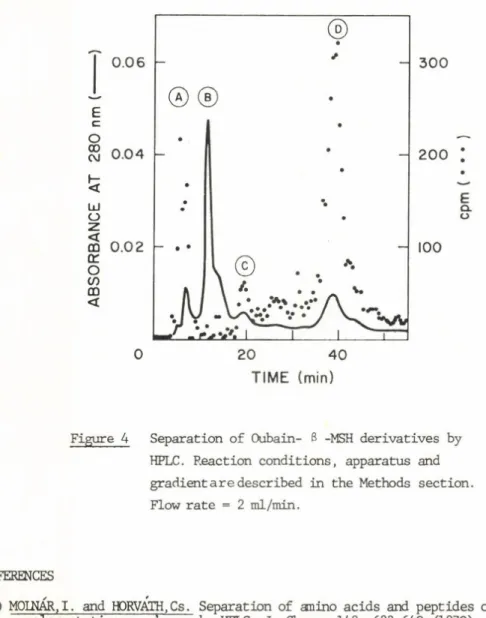
3) Ouabain-ß-MSH
Ouabain was linked to ß-MSH through aldehyde groups formed by period
a te oxidation of the deoxymannopyranosyl group of the molecule. Since both the 3-4 and 4-5 carbon bonds of the carbohydrate can be cleaved by per
iodate oxidation, four d ifferen t aldehydes can be formed which may react with the three free amino groups of ß-MSH. The sim plicity of the HPLC elution pattern (Figure 4) may be a consequence of an incomplete resolution
2* 9
♦
of the numerous stru c tu ra lly sim ilar ouabain-g-MSH derivatives; a lte r n a ti
vely, few major reaction products could have been formed. Peak A is
probably a polymerized product of oxidized ouabain; peak B is native g-MSH;
peaks C and D are ouabain-g-MSH conjugates. The tyrosinase-stim ulatory a c tiv ity of fraction (D) is 66% of th at of the unsubstituted g-MSH (10-^M).
Surprisingly, while free ouabain (1 pM) was highly toxic to melanana c e lls, ouabain-g-MSH (peak D) stimulated growth. When ouabain41SH was added (0.1 pM) to melanoma c e ll cultures during the f i r s t day of cultivation, c e lls grow about twice as fa s t as untreated c e lls. Whether th is stimulation is related to an interaction between receptors for MSH and an Na+/K+ pump in the c e ll membrane, is under investigation.
CONCLUSIONS
As we have shown, RPLC is a convenient method for separating and purifying amino substituted derivatives of g-MSH, In some cases well- resolved homogenous fractions can be obtained in one run. In others, re - chrcmatography is needed to resolve stru ctu ra lly sim ilar biologically active peptide derivatives. On RPLC, amino-substituted derivatives of g-MSH appear la te r than the unsubstituted peptide. The retardation of the conjugates is probably due to an increased hydropbobicity because the peptide derivatives are formed by the substitution of ionizable amino groups with re la tiv e ly large arcmatic or saturated cyclic compounds (1,9).
ACKNOWLEDGMENTS
We thank Dr. Saul Lande fo r providing the g-MSH, Noel Richard and Tracy Bobak for th e ir help with the tyrosinase assays, Dr. D. Lambert for
setting up the Waters HPLC system, Dr. Gisela Moellmann for correcting and Elena DiMassa for typing the manuscript. This investigation was supported by Grant Number R01-CA-26081 awarded by the National Cancer In s titu te , DHEW.
10
3 0 0
200
100
0 2 0 4 0
TIME (min)
ECL
O
Figure 4 Separation of Oubain- 3 -MSH derivatives by HPLC. Reaction conditions, apparatus and gradient a r e described in the Methods section.
Flow ra te = 2 ml/min.
REFERENCES
(1) MOLNÁR, I . and HORVATH,Cs. Separation of amino acids and peptides on non- polar stationary phases by HFLC. J . Chrom. 142: 623-640 (1970)
(2) Proceedings of the International Symposium on HPLC of Proteins and Peptides 1981, Washington, D.C. J.Anal.Biochem. 1982.
(3) ASATON., LANDE,S., VARGA,J.M., MOLNÁR,I. and HORVATH,Cs. Increase of biological a c tiv ity of a ß-MSH fragment a fte r conjugation with
ty ro sin e-lik e compounds. Yale J.Biol.Med. 50: 546 (1977)
(4) LAMBERT,D.T., STACHLEK C. , VARGA, J.M. and LERNER.A.B. Iodination of - MSH: time course analysis of reaction mixtures by HPLC and
characterization of biologically active mono- and d i- iodo ß^iSH.
J.B iol. Chem. 257: 8211 (1982)
(5) PCMERANTZ,S.H. L-tyrosine 3,5 assay fo r tyrosinase development in skin of newborn hamsters. Science 164 : 838 (1969)
(6) VARGA,J.M. and ASATO.N. , LANDE,S. an3TLERNER,A.B. Melanotropin-
dauncmycin conjugate shows receptor-mediated cytotoxicity in cultured murine melanoma c e lls . Nature 267: 56-58 (1977)
11
(7) VARGA, J.M. , MOELLMANN.G. , FRITSCH,P. , GODAWSKA.E. and LERNER.A. B.
Association of c e ll surface receptors for melanotropin with the Golgi region in mouse melanoma c e lls . Proc.Nat.Acad.S ei.USA 73: 559-562
(1976)
(8) GARLSSON.J., PREVIN,H. and AXEN,R. Protein th io la tio n and reversible protein-protein conjugation. N-Succin-imidyl 3-(2-Pyridyldithio) propionate a new heterobifunctional reagent. Biochem.J. 173 : 723 -737,
(1978)
(9) MF.KK,J.L. Prediction of peptide reten tio n times in high-pressure liquid chromatography on the basis of amino acid composition. Proc.Nat.Acad.
Sei. USA 77: 1632-1636 (1980)
12
Pr oa , o f t h e Symp. on A d v a n c e s i n L i q u i d C h r o m a t o g r . , S z e g e d , H u n g a r y, 1982
H I G H -P E R F O R M A N C E L I Q U I D CHROMATOGRAPHY OF F I B R O U S P R O T E I N S
Z , D E Y L , K, MACEK
Physiological Institute, Czechoslovak, Academy of Sciences, Prague, Czechoslovakia
In protein separation i t is possible to exploit the molecular size, charge differences and the hydrophobicity of species to be separated. According to these properties i t is possible to use gel permeation chromatography, ion exchange chromatography or reversed phase HFLC. Each of these techniques has i t s own advantages and lim itations. Thus, for instance, reversed phase chromatography can be used only with proteins which re ta in th e ir so lu b ility in re la tiv e ly hydrophobic media and which, i f possible, can be recovered in native sta te a fte r some additional operation. Therefore, reversed phase
chromatography has been used mainly with low-molecular weight peptides and proteins and the other two techniques mentioned above are s t i l l prevailing in the separations of giant molecules.
A category of fa r the larg est protein molecules is represented by fibrous proteins. Only the category of collagen has been approached by modem chromatographic techniques (1,2) while the others lik e fibrous proteins of muscle, r e s ilin , fibroin, etc. are at the moment neglected completely. Col
lagen separations are tra d itio n a lly a d ifferen t problem as long as collagen represents a family of very closely related six d ifferen t proteins of r e la tiv e ly low s o lu tib ility th at may occur side by side in tissu es. From the separation point of view collagens represent a rare category of very high- molecular proteins in which a l l the three separation p rin cip les, i .e ., gel permeation, ion exchange and reversed phase p a rtitio n can be applied. Some of the members of th is family (individual collagen types) d iffe r in th e ir re la tiv e molecular mass, others in th e ir charge or hydrophobicity, some have a s tr ic tly rig id h elical structure while in others interruptions of th is structure by flex ib le regions are seen. (For review see. r e f . 3). Therefore th is category of proteins represents an excellent model on which the app li
13
c a b ility of d ifferen t chromatographic principles can be demonstrated. (For review see r e f .4).
At the beginning of our studies using HPLC we attempted to separate collagen type I and I I I polypeptide chains using a copolymer of 2-hydroxy- ethyl methacrylate with ethylene dimethacrylate covalently coated with glucose (Separon HEMA 1000 Glc gel) (F ig.la, b ) .
Fig.l a . The chromatographic p ro file of a mixture of collagen polypeptide chains. Type I I I collagen is pres
ent in i t s disulphide bonded form a 1(III) 3
Fig.l b . The chromatographic pro
f i l e of a mixture of collagen polypeptide chains. In the mixture disulphide bonds were cleaved by oxidation p rio r to chromatography and therefore type I I I collagen is present in i t s monomeric form as
cxl (III)
14
Though a good quality of separations was achieved, the process was not gov
erned solely by gel permeation, as long as molecular e n titie s of identical re la tiv e molecular mass were separated. This may be of advantage in the sep
aration of certain collagen mixtures, but causes considerable d if fic u ltie s when gel permeation seoarations are used for the investigation of complex mixtures of d ifferen t collagen polypeptide chains and th e ir fragments. We attempted therefore to abolish the secondary interactions as much as poss
ib le and establish a high performance procedure in which gel permeation would be the only mechanism involved.
EXPERIMENTAL
Separations were done on a Pye Unicam liquid chromatograph LC 20 equipped with the UV spectrophotometric detector LC-3 set a t 230 nm. A stain less s te e l column (500 x 8 nm) prepacked with Separon HEMA 1000 Glc, p a rtic le size 12-17 /m, was used. The apparatus was operated a t about 1'5 mPa overpressure which gave a flow ra te of 1.5 ml/min. Of the several mobile phases tested the best resolutions were obtained by iso cratic elution with a solution composed of 0.2 mol/L NaCl - 2 mol/L urea - 0.05 mol/L T ris. HCL buffer, pH 7.5. Samples of individual collagen types and th e ir fragments were prepared by established methods. In order to avoid problems arising from UV absorbency of mercaptoethanol, disu lfide bonds cleavage was done by performic acid treatment.
RESULT'S AND DISCUSSION
The ap p licab ility of the worked-out procedure is demonstrated on the following examples. Clearcut separations are obtained with alpha-chain polymers (Fig.2) and a rapid information can be obtained about S-S bond cleavage in collagen type I I I (Fig.3). The separation efficiency is s u f f i
cient to distinguish between alpha^ (IV) and a l p t ^ (IV) collagen poly
peptide chains, a re s u lt that has not been visualized by gel permeation chromatography before (Fig.4).
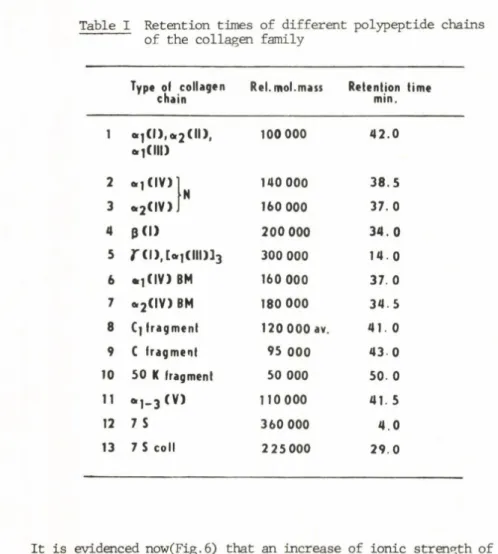
A general image about the retention times of individual collagen species and some of th e ir fragments is summarized in Table 1. The retention times decrease with increasing re la tiv e molecular mass, however,the decrease is not s tr ic tly linear in the logarithmic scale as i t would be expected (Fig.5). No separation of collagen polypeptide chains of id entical re la tiv e
15
molecular mass but originating fron d ifferen t collagen types was observed.
I t has been communicated before th a t sorption plays an inportant ro le in high performance gel permeation chromatography. Indeed in our previous pa
pers we have observed the separation of, e .g ., alpha-^(I) and alpha^(III) on the same sorbent th at has been used in the present experiments when iso cratic elution with 0.05 M Tris-HCl buffer, pH 7.5 (2M with respect to urea) was used. This was ascribed eith er to differences in hydrodynamic volumes of matching collagen peptide chains originating from d ifferen t species or adsorption and/or p a rtitio n interactions of the separated protein molecules, or fin a lly to the possible weak a ffin ity of collagen chains to the glucose coated macroporous adsorbent causing respective retentio n differences of otherwise sim ilar molecules.
“ (I)
F ig,2 GPC separation of a-chain polymers with the mol
ecular sieving effect as the governing mechanism of separation
16
Fig.3 GPC of collagen type I I I In i t s trim eric (upper panel) and monomeric form (lower panel, a fte r S-S bond cleavage)
17
Rel. mol. mass (KD)
- 2 (IV)
0 4 8 12 16 20 24 2 8 32 36 4 0 4 4 48 52 min Fig.4 Separation efficiency of the HPGPC procedure demonstrated
by the p a rtia l separation of both collagen type IV ct—chains
Retention time (min)
F ig.5 Log molecular mass vs. retention time p lo t of the collagen chains showing n on-linearity of the rela tio n
18
Table I Retention times of different polypeptide chains of the collagen family
Ty p e o l c o l l a g e n c h a i n
R e l . m o l . m a s s R e t e n t i o n t i m e m i n .
1 e i ] ( l ) , o i e . l ( l l l )
2 < « > . 1 0 0 0 0 0 4 2 . 0
2 01] ( I V ) N
1 4 0 0 0 0 3 8 . 5
3 o 2 ( I V ) 1 6 0 0 0 0 3 7 . 0
4 0 ( 1 ) 2 0 0 0 0 0 3 4 . 0
5 r ( i ) , c o . ] ( i i i ) 3 3 3 0 0 0 0 0 1 4 0
6 • ] ( I V ) BM 1 6 0 0 0 0 3 7 . 0
7 Or2 ( I V ) BM 1 8 0 0 0 0 3 4 . 5
8 C] f r a g m e n t 1 2 0 0 0 0 av. 4 1 0
9 ( f r a g m e n t 9 5 0 0 0 4 3 0
1 0 5 0 K f r a g m e n t 5 0 0 0 0 5 0 0
11 o , _ 3 ( V ) 1 1 0 0 0 0 4 1 . 5
12 7 S 3 6 0 0 0 0 4 . 0
1 3 7 S c o l l 2 2 5 0 0 0 2 9 . 0
I t is evidenced now(Fig.6) th at an increase of ionic strength of the eluent is capable of completely abolishing the inter-species differences at le a st between collagen I, I I I , IV and V. The same re s u lt can be achieved eith er by adding NaCl to the eluting solvent or by increasing the concentra
tion of the Tris buffer to 0.5 Mol/L. The separation conditions can be se
lected in such a way that gel permeation is the only mechanism governing the separation. S t i l l the non-linearity of the reten tion time vs. logarithm of molecular mass re la tio n indicates th at some other effects though minimalized s t i l l p e rs is t throughout the separation effected on Separon MEHA 1000 Glc.
19
Fig-6 Dependency of the retention time upon the composition of the mobile phase
In other words with th is category of gels i t is possible to make a ratio n al selection of conditions th a t would o ffer separations eith er solely on the basis of molecular sieving or may exploit other interactions as well offering thus separations of protein species of id entical re la tiv e molecular mass. In our opinion th is widens the separation p o s s ib ilitie s not only with collagen proteins but with other protein mixtures as well.
20
REFERENCES
(1) FALON,A., LEWIS,R.V. and GIBSON,K.D. : Anal.Biochem. 318 (1981) (2) MACEK.K., DEYL.Z., COUPEK.J. and SANITRAK,J.: J.Chromatogr.,222
284 (1981)
(3) GIANVILLE.R.W. in Conn. Tiss.R es., Chemistry, Biology and Physiology, Z.Deyl and M. Adam Eds, Alan R. Liss Inc. New York 1981, gp.1-14.
(4) DEYL.Z., HORAKOVAjM. and ADAM,M. in Conn.Tiss.Res., Chemistry, Biology and Physiology, Z.Deyl and M. Adam Eds, Alan R.Liss Inc.New York 1981, gp. 15-44.
P r o a , o f t h e Symp. on A d v a n c e s i n L i q u i d C h r o m a t o g r . , S z e g e d , Hu n g a r y , 1982
A D S O R P T I O N CHROMATOGRAPHY OF F L E X I B L E POLYMER MOLECULES
G. GLÖCKNER
Department of Chemistry, Technical University of Dresden, Dresden, GDR
SUMMARY
TLC i n v e s t i g a t i o n o f v a r io u s polym ers i n d i c a t e s r e v e r s i b l e a d s o r p tio n . The n o n - l i n e a r i t y o f polym er a d s o r p tio n is o th e rm s i s due to a c o o p e r a tiv e e f f e c t . In column AC, th e e l u t i o n volume o f p o l y ( s t y r e n e - c o - a c r y l o n i t r i l e ) sam ples was found to v a ry a c c o rd in g to th e a c r y l o n i t r i l e c o n te n t.
INTRODUCTION
As e a r l y as 1936, MARK and SAITO (1) f i l t r a t e d s o lu ti o n s o f c e l l u l o s e a c e t a t e in a c e to n e th ro u g h columns packed w ith c h a r c o a l and i s o l a t e d f r a c t i o n s from d i s i n t e g r a t e d zones o f th e p a ck in g by e x t r a c t i n g w ith d io x a n e . But o n ly a few p a p e rs d e a l in g w ith th e a d s o r p tio n chrom atography (AC) o f polym ers were p u b lis h e d d u rin g a p e r io d o f ab o u t 30 y e a rs a f t e r t h i s in c e p t i o n . In 1968, BELENKIJ and GANKINA (2) as w e ll as INAGAKI (3) in d e p e n d e n tly perform ed t h i n - l a y e r ch ro m a to g rap h ic s e p a r a tio n s o f co p olym ers. T h e ir p u b l i c a t i o n s s tim u la te d a g r e a t number o f s u c c e s s f u l TLC i n v e s t i g a t i o n s o f v a r io u s problem s c o n c e rn in g polym er s i z e , c o m p o sitio n , and a r c h i t e c t u r e . D uring th e l a s t d eca d e, numerous e x c e l l e n t b a s e li n e s e p a r a t io n s o f o lig o m ers were a c h ie v e d by means o f h ig h p erfo rm an ce column chrom atography u s in g e i t h e r r e v e r s e d ph ases (4 -1 3 ) o r b a re s i l i c a (1 4 -2 1 ) as a p ack in g m a t e r i a l . S e p a r a tio n s o f t h i s k in d w ere a c h ie v e d f o r lo w -m o le c u la r p o ly - s ty r e n e (4 , 5, 6, 14, 15, 16) and p o ly (m e th y l m e th a c r y la te ) (1 7 ), f o r p o ly ( e th y le n e o x id e ) (7 , 8, 9 ), p o ly -
- ( e th y l e n e t e r e p h t b a l a t e ) (18, 1 9 ), polyam ide 6 (2 0 ), f o r epoxy r e s i n s (10, 11, 1 2 ), and p o l y e s t e r s (1 3 ). KLEIN and LEIDIGKEIT
(21) even s p l i t up a m ix tu re o f some p o ly s ty r e n e (PS) sam ples
3 23
(w ith m olar mass v a lu e s ra n g in g betw een 2000 and 2000,000 g/m ole) by means o f AC.
These r e s u l t s a r e r e a l l y re m a rk a b le b eca u se th e a d s o r p tio n is o th e rm s o f polym ers a r e known to be e x tre m e ly n o n - l i n e a r . They e x h i b i t a p s e u d o p la te a u r e g io n w hich v i r t u a l l y co v ers th e whole c o n c e n tr a tio n ran g e o f i n t e r e s t . C a lc u la tio n s perfo rm ed by FLEER and SCHEUTJENS (22) d e m o n stra te t h a t th e p s e u d o p la te a u e x te n d s to v e ry low c o n c e n tr a tio n s . I t s t r a n s i t i o n i n t o th e Henry r e g ion (w ith th e amount a d so rb ed p r o p o r ti o n a l to th e c o n c e n tr a tio n o f th e s o lu ti o n ) o c c u rs a t a c o n c e n tr a tio n w hich i s th e s m a lle r th e h ig h e r th e m olar mass o f th e p olym er. For c h a in s o f o n ly a hundred segm ents, th e c r i t i c a l volume f r a c t i o n i s even as sm all as 10 ' . T his means t h a t s o lu ti o n s w hich c o n ta in more th a n one -25 c h a in m o lecu le in ab ou t 100 1 s o lv e n t e x h i b i t an a d s o r p tio n which i s n e a r l y i n s e n s i t i v e to a change in c o n c e n tr a tio n . At a v ery low c o n c e n tr a tio n , th e a d s o r p tio n c o n s ta n t i s e x tre m e ly la r g e , b u t in th e norm al ra n g e o f c o n c e n tr a tio n s i t s d i f f e r e n t i a l v a lu e i s n o t f a r from z e ro . As o n ly a f i n i t e v a lu e o f th e d i s t r i b u t i o n c o n s ta n t w hich does n o t v a ry to o much w ith c o n c e n tr a tio n e n a b le s th e ch ro m a to g rap h ic p ro c e s s to be perform ed in a common m anner, th e AC o f polym ers w i l l c e r t a i n l y n o t work un d er c o n d itio n s em
p lo y ed in s t a t i c a d s o r p tio n m easurem ents. Only u n d er c a r e f u l l y s e le c te d c o n d itio n s can r e a s o n a b le v a lu e s o f polym er r e t e n t i o n be re a c h e d . The a d s o r p tio n energy o f th e s o lu te must be b a la n c e d w ith th e a d s o r p tio n en ergy o f th e s o lv e n t, and th e s o l v e n t / s o l u t e
i n t e r a c t i o n en erg y .
INVESTIGATION INTO THE BEHAVIOUR OF MACROMOLECULES IN ADSORPTION CHROMATOGRAPHY
Tne b e h a v io u r o f lo w -m o lecu la r s o lu te s in AC can be d e s c rib e d u s in g th e e q u a tio n g iv e n by SNYDER (2 3 ):
lo g K+ = lo g Va + a A(S° - Ag . e ° ) (1) K+ : a d s o r p tio n c o n s ta n t o f th e s o lu te
Va : volume o f a m onom olecular la y e r o f th e s o lv e n t on th e s u r f a c e o f th e a d s o rb e n t ( " s u r f a c e volum e")
24
a A: a c t i v i t y o f th e a d s o rb e n t < 1 S° : a d s o r p tio n en erg y o f th e s o l u t e
Ag: s u r f a c e a r e a co vered by a s o lu te m o lecu le
e ° : a d s o r p tio n en erg y o f th e s o lv e n t
Among th e p r e r e q u i s i t e s f o r t h i s fo rm u la i s th e need f o r th e r e v e r s i b i l i t y o f th e a d s o r p tio n p r o c e s s . I f i t can be proved t h a t th e SNYDER e q u a tio n a ls o h o ld s t r u e f o r polym ers t h i s would in d i c a t e t h a t m acrom olecules a r e r e v e r s i b l y f ix e d un d er th e con
d i t i o n s o f AC. In o r d e r to perfo rm t h i s t e s t , we looked f o r c i r cum stances c a p a b le o f b a la n c in g K a g a i n s t V . The s u r f a c e vo-
-2 3 a
lume i s ab o u t 10 cm / g. C o n seq u en tly , th e a d s o r p tio n c o e f f i c i e n t must a ls o be s m a ll. In TLC m easurem ents, t h i s c a l l s f o r h ig h v a lu e s . We choose R£ = 0 .7 , w hich i s c e r t a i n l y s t i l l on th e s a f e s id e as co n cern s th e volume p r o f i l e on th e p l a t e . In TLC w ith p u re s o lv e n t s , polym ers e i t h e r rem ain a t th e s t a r t i n g sp o t o r ru n w ith th e s o lv e n t f r o n t . B in a ry m ix tu re s o f a s tro n g s o lv e n t (R£ = 1) and a weak one (R£ = 0) a ls o e x h i b i t th e one o r th e o th e r ex trem e b e h a v io u r in n e a r l y th e whole ran g e o f p o s s i b le c o m p o sitio n s . The t r a n s i t i o n from R^ = 0 to R^ = 1 ta k e s p la c e in a r a t h e r n arrow ra n g e . We i n v e s t i g a t e d 6 p o ly mers in 3 co m b in atio n s o f s tr o n g and weak s o lv e n ts and o b ta in e d
th e r e s u l t s shown in F ig . 1 (2 4 ). The a b s c is s a i n d i c a t e s th e
e M v a lu e o f th e m ix tu re as c a l c u l a t e d from th e c o m p o sitio n and
e 0 v a lu e s o f th e p u re com ponents. The w hich co rresp o n d ed to R£ = 0 .7 were e v a lu a te d by i n t e r p o l a t i o n from th e s e c u rv e s . These e x p e rim e n ta l d a ta were av erag ed s e p a r a t e l y f o r each i n d iv id u a l polym er and p l o t t e d v s . S°/Ag. The l a t t e r were c a l c u l a t e d f o r th e r e s p e c t i v e polym er by th e in c re m e n ts g iv e n by SNYDER (2 3 ). The s tr a i g h tf o r w a r d p l o t t i n g scheme fo llo w s from Eq. (1) i f lo g K+= lo g V •
a.
0 = a A(S° - As . e ° ) (2)
3* 25
F ig . 1
TLC r e s u l t s from 6 polym ers on s i l i c a u s in g th e b i n a r i e s te tra c h lo r o m e th a n e /d io x a n e (o) to lu e n e /a c e to n e ( • ) , and t o l u e n e / e t h y l a c e t a t e
( x ) . PC: p o ly c a rb o n a te ; PBMA: p o ly ( b u ty l m etha
c r y l a t e ) ; a MSAN: p o ly ( a m e th y l- s ty r e n e - c o - - a c r y l o n i t r i l e ) , ab o u t 46 mole °L AN; SAN: p o ly - - ( s t y r e n e - c o - a c r y l o n i t r i l e ) , abou t 38 mole °L AN; PMMA: p o ly (m e th y l m e th a c r y la te ) ; CA: c e l l u lo s e t r i a c e t a t e (2 4 ).
In t h i s c a s e , th e a c t i v i t y a ^ o f th e a d s o rb e n t does n o t m a t
t e r , and th e S°/As v a lu e s can be c a l c u l a t e d f o r an a r b i t r a r y p a r t o f th e polym er c h a in , f o r in s ta n c e sim ply f o r one r e p e a tin g u n i t . The s t r a i g h t - l i n e in F ig . 2 i n d i c a t e s th e e q u a l i t y o f S°/Ag and e M> fo llo w in g from th e SNYDER e q u a tio n u n d er th e c o n d itio n g iv e n in Eq. ( 2 ) .
F ig . 2
E v a lu a tio n o f r e s u l t s from F ig . 1 a c c o rd in g to Eq. ( 2 ) . The p o in ts c o r resp o n d to th e polym ers PS, PC, PBMA, a MSAN, SAN, PMMA, CA (from l e f t to r i g h t ) (29)
26
The p o in t s o b v io u s ly a g re e w ith t h i s l i n e , th u s c o n firm in g th e SNYDER fo rm u la as w e ll as th e r e v e r s i b i l i t y o f th e a d s o r p tio n . Modern th e o r i e s o f polym er a d s o r p tio n a ls o s t a t e r e v e r s i b i l i t y , though th e m acrom olecules c a n n o t be removed from th e s u r f a c e by d i l u t i o n . What i s r e v e r s i b l e i s th e a d s o r p tio n p ro c e s s o f any s i n g l e segm ent. L inked to i t s n e ig h b o u rs , i t can n o t escap e from th e s u r f a c e and w i l l more l i k e l y be re a d s o rb e d th a n b ein g so c lo s e to th e s u r f a c e u n t i l th e o th e r segm ents o f th e ad so rb ed t r a i n a r e e v e n t u a ll y a l s o d e so rb e d . I t i s a k in d o f c o o p e r a tiv e e f f e c t w hich makes a m acrom olecule u n a b le to le a v e th e a d s o r p t i o n l a y e r . On th e o th e r hand, i f m acrom olecules a r e c a p a b le o f m ig r a tio n in AC te c h n iq u e th e y w i l l c e r t a i n l y n o t to u c h th e
s u r f a c e w ith a g r e a t many segm ents. In o r d e r to i n v e s t i g a t e t h i s , we r e - a r r a n g e d Eq. ( 1 ) . :
In K = - - H = 2 .3 lo g (K*/V ) = 2.3 a A (S° - A „. e ° ) (3)
kT a A b
The d e r i v a t i v e r e a d s : d ln K
d e o
2 . 3 a a • Ag (4)
F ig u re 3 r e p r e s e n t s e x p e rim e n ta l r e s u l t s w hich BELENKIJ e t a l . (25) have o b ta in e d i n v e s t i g a t i n g p o ly s ty r e n e s ta n d a r d s by means o f TLC on s i l i c a w ith d e v e lo p in g m ix tu re s o f c y c lo h e x a n e , b en zen e , and a c e to n e . The c o n te n t o f th e s tr o n g s o lv e n t a c e to n e was v a r ie d from 1 .5 to 2 .8 p a r t s in m ix tu re s w ith 56 p a r t o f th e w eaker com ponents. At h ig h v a lu e s o f e t h e r e i s no a d s o r p t i o n , and th e m ig r a tio n o f th e v a r io u s PS p ro b es i s governed by an e x c lu s io n mechanism. The AC ta k e s p la c e in th e r e g io n below th e i n t e r s e c t i o n p o in t a t e ^ = 0 .2 4 6 . H ere, th e e x p e r im e n ta l r e s u l t s can be r e p r e s e n te d by s t r a i g h t l i n e s , th e s lo p e o f w hich g iv e s th e d e r i v a t i v e e x p re s s e d by Eq. ( 4 ) . (For n u m e ric a l e v a l u a ti o n , th e p r o p o r t i o n a l i t y e g^Q = 0 .7 7 £ ^ q
m ust be ta k e n i n t o a c c o u n t.)
27
W kT
F ig .3 .
TLC r e s u l t s o b ta in e d w ith 5 PS S ta n d a rd s u s in g mixed e lu e n ts on s i l i c a . P lo t o f - l n K = A F/kT v s . e ^ A l^ O n ) , a c c o rd in g to BELENKIJ e t a f .
(25)
These d a ta and th e m olar mass o f th e sam ples a r e com piled in T ab le 1. For a f i r s t a p p ro x im a tio n we to o k = 1. Thus th e d a ta in column 2 can be u n d e rs to o d as th e s u r f a c e a r e a co v ered by th e segm ents o f one m acro m o lecu le. For one s ty r e n e u n i t , Ag amounts to 6 .7 . The f i g u r e s in th e t h i r d column i n d i c a t e how o f te n 6.7 i s c o n ta in e d in th e Ag v a lu e o f th e m ig r a tin g PS sam ple.
T ab le 1
S lope o f th e s t r a i g h t l i n e s in F ig . 3, and c o n c lu s io n s M
g/m ole
d( A F/kT) c o n ta c tin g s ty r e n e u n i t s p e r c h a in
s ty r e n e u n i t s p e r c o n ta c t 2 - 3 d e S i0 2
19600 6.35 1 200
49000 19.1 3 165
96200 35.6 5 175
164000 57.6 9 185
392000 158.2 24 160
From t h i s ro ugh a p p ro x im a tio n , i t can be co n clu d ed t h a t m acro
m o le c u le s a r e a b so rb ed w ith no more th a n 1 ?0 o f t h e i r r e p e a tin g u n i t s un d er th e c o n d itio n s o f AC.
28
SEPARATION OF COPOLYMERS ACCORDING TO CHEMICAL COMPOSITION S e p a r a tio n a c c o rd in g to m olar mass w i l l c e r t a i n l y n o t become th e main ta s k f o r polym er AC, b ecau se h e re th e r e a r e e f f e c t i v e c o m p e titiv e m ethods, as f o r in s ta n c e SEC. The chem ical com posi
t i o n d i s t r i b u t i o n (CCD) o f copolym ers has been an im p o rta n t aim o f many TLC i n v e s t i g a t i o n s . By column AC on s i l i c a , TERAMACHI e t a l . (26) f r a c t i o n a t e d copolym ers o f s ty r e n e and m eth y l a c r y l a t e (MA). They found r e t e n t i o n in c r e a s in g w ith MA c o n te n t u s in g a l i n e a r g r a d ie n t o f m ethy l a c e t a t e (7 to 35 vol.% ) in CC1,. Employing i n t e r n a l s ta n d a r d s , th e a u th o rs w ere a b le to c o n v e rt th e e l u t i o n cu rv e in t o th e cu rv e o f CCD, b u t in r e p e a te d ex p e rim e n ts a l l t h r e e copolym er sam ples (4 6 .6 ; 5 7 .3 ; 7 7 .9 mole
% MA) were m o stly e l u te d w ith a low er c o n te n t o f m ethy l a c e t a t e th a n in th e p re c e d in g r u n s . DANIFLEWICZ and KUBIN (27) i n v e s t i g a te d p o ly ( s ty r e n e - c o - m e th a c r y la te ) sam p les. They employed a g r a d ie n t o f te tr a h y d r o f u r a n (THF, 3 to 20 %) in d ic h lo r o ethylene.
A f te r each ru n , th e y f lu s h e d th e s i l i c a in th e column by a t l e a s t 10 column volumes o f p u re THF and o b ta in e d r e p r o d u c ib le r e t e n t i o n v a lu e s . Block copolym ers o f s ty r e n e and m ethyl m e th a c r y la te were i n v e s t i g a t e d by BELENKIJ (2 8 ). The a b s o rb e n t was s i l i c a , th e s o lv e n t d ic h lo r o m ethane w ith in c r e a s in g amounts o f m ethanol added.
We investigated the behaviour of poly(styrene-co-acrylo- nitrile) in a silica column with THF in hexane.
MATERIAL AND METHODS
C opolym ers: The specim ens w ere p o ly m erize d in b u lk w ith azo b i - s i s o b u t y r o n i t r i l e as an i n i t i a t o r . The p o ly m e r iz a tio n s were perfo rm ed in s e a le d am poules w hich were shaken in a w a te r b a th o f 60 °C. A f te r c o n v e rs io n up to 5 - 10 %, th e r e a c t i o n was sto p p e d by c o o lin g th e am poules and p u rin g th e r e a c t i o n m ix tu re i n t o s t i r r e d m e th an o l. A ll th e polym ers r e - p r e c i p i t a t e d tw ic e and d r ie d u n d er vacuum a t 60 °C. T able 2 g iv e s d a ta o f th e s p e cim ens u se d .
29
T able 2
S t y r e n e / a c r y l o n i t r i l e copolym ers i n v e s t i g a t e d
; ample a c r y l o n i t r i l e
c o n te n t M
osm
7o p e r mole g/moli
D68 7 .9 244000
D47 2 7.4 325000
D55 37.8 297000
D38 5 9 .4 340000
S o lv e n t s : T e tr a h y d r o f u r a n , a n a l y t i c a l l y p u re , was s to r e d w ith KOH f o r 24 h o u rs , r e f lu x e d w ith Na f o r 2 h o u rs and d i s t i l l e d . n-H exane, a . p . , a s s u p p lie d by E. MERCK, D arm stad t.
For u se as e l u e n t s , b o th s o lv e n ts were mixed w ith 0 .5 °L m e th a n o l.
HPLC a p p a r a tu s : HP 1080A (H ew lett P ack ard) w ith UV d e t e c to r (254 nm ). Column: 0 ,2 5 m lo n g , 4 .6 mm I .D . , w ith L i C h r o s p h e r ^ SI 100, dp = 10 jam. Flow r a t e : 2 m l/m in. G ra d ie n t e l u t i o n w ith THF (20 to 90 7o) in hexane.
Sample i n j e c t i o n : 50 ;u l o f s o lu ti o n s c o n ta in in g 0 .4 g /1 p o ly mer in THF.
RESULTS
The chrom atogram s u s u a ll y s t a r t e d w ith two sh arp p e a k s , th e f i r s t o f w hich was due to some u n r e ta in e d p olym er, th e o th e r to th e i n j e c t i n g s o lv e n t . The e l u t i o n volume o f th e r e t a i n e d polym er v a r i e d a c c o rd in g to i t s a c r y l o n i t r i l e c o n te n t. F ig . 4 g iv e s some exam ples. As hexane i s a n o n - s o lv e n t f o r th e copolym ers in v e s t i g a t e d , th e ru n s began w ith th e p r e c i p i t a t i o n o f th e polym er.
T his happened w ith o u t b u ild in g up an a d d i t i o n a l flow r e s i s t a n c e . The p r e s s u r e change d u rin g th e ru n s o n ly r e p r e s e n te d th e in c r e a s e in v i s c o s i t y due to th e t r a n s i t i o n from 20 to 90 °L THF. T here was no in f lu e n c e from th e polym er i n j e c t e d .
30
F ig . 4
UV r e c o r d a t 254 nm a f t e r i n j e c t i o n o f 20 j/ig SAN c o polym er .
Upmost t r a c e : a c e o tr o p ic copolym er w ith 37 .8 % AN, o t h e r s : m ix tu re s o f two sam ples each , AN c o n te n t in d i c a te d
The d e l iv e r y o f a c e r t a i n polym er specim en from th e column alw ays to o k p la c e a t a c e r t a i n THF c o n te n t w hich c o rre sp o n d e d to th e THF c o n te n t in a p r e c i p i t a t i n g m ix tu re . From t h i s i t can be co n cluded t h a t th e mechanism i s s o l u b i l i t y d e term in e d
DISCUSSION
A dsorbed polym ers can n o t be removed from th e s u r f a c e by d i l u t i o n , though th e a d s o r p tio n i s r e v e r s i b l e f o r any segm ent. The d e s o rp t i o n o f m acrom olecules w i l l e a s i l y advance i f a com peting su b s ta n c e w ith a h ig h e r l e v e l o f a d s o r p tio n en erg y i s added. T his s u b s ta n c e w i l l ta k e o v er ev ery a d s o r p tio n s i t e as soon as i t i s l e f t by th e polym er segment f o r a moment. So ta k in g ad v an tag e o f th e dynam ics o f a d s o r p tio n and d e s o r p tio n s t e p s , th e s tr o n g e r s o lv e n t o r polym er d is p la c e s th e s p e c ie s a d s o rb e d . From t h i s i t can be deduced t h a t g r a d ie n t e l u t i o n sh o u ld be th e p r e f e r r e d mode o f polym er a d s o r p tio n ch ro m atography. In d eed , t h i s i s c l e a r
ly r e f l e c t e d by th e l i t e r a t u r e .
In some TLC work, no e f f o r t was ta k e n to produ ce any g r a d i e n t , b u t t h i s need n o t mean t h a t th e s e p a r a tio n p ro cee d ed i s o -
31
c r a t i c a l l y . In TLC w ith mixed e lu e n ts th e r e a r e s e v e r a l p o s s i b i l i t i e s o f e s t a b l i s h i n g a sp o n tan eo u s g r a d ie n t: v ia th e vapour p h a s e , by dem ixing d u rin g th e ru n due to d i f f e r e n t v e l o c i t y ch a r a c t e r i s t i c s , o r a c c o rd in g to a d i f f e r e n c e in e l u o t r o p i c strength Such a h id d e n g r a d ie n t can be r a t h e r m e a n in g fu l in polym er s tu d i e s . In th e l i t e r a t u r e th e r e a r e r e p o r t s on th e f a i l u r e o f i s o - c r a t i c column AC r u n s , w hich were seem in g ly perfo rm ed u n d er th e same c o n d itio n s as s u c c e s s f u l TLC s e p a r a t io n s .
T ru ly i s o c r a t i c a d s o r p tio n chrom atography o f polym ers can o n ly work on c o n d itio n o f a n e a r l y com plete b a la n c e betw een a d s o r p tio n f o r c e s and s o lv in g power. F in e examples o f such d if f ic u lt work a r e th e o lig o m er s e p a r a tio n s r e p o r te d by EISENBEISS e t a l .
(15) and by KNOX and McLENNAN (1 6 ).
REFERENCES
(1) MARK, H .; SAITO, G .(1936) M onatsch. Chemie 68 237-243 (2) BELENKIJ, B .G ., GANKINA, E .S . (1969) Doklady Akademii Nauk
SSSR 186 857
(3 % INAGAKI, H .. MATSUDA, H ., KAMIYAMA, F (1968) M acromole- c u l e s _ 1 520-525
(4) KIRKLAND, J . J . (1975) C hrom atographia 8 661-668 (5) PARRIS, N.A. (1978) 157 161-170
(6) LATTIMER, R .P ., HARMON, D .J ., WELCH, K.R. (1979) A n a ly t.
Chem. 51, 1293-1296
(7) MELANDER, W.R., NAHUM, A ., HORVATH, Cs. (1979) J . Chroma- t o g r . 185 129-152
(8) NOZAWA, A ., OHNUMA, T. (1980) J . C h rom atogr. 1_87 261-263 (9) MURPHY, R ., SELDEN, A .C ., FISHER, M ., FAGAN, E .A ., CHAD
WICK, V.S. (1981) J . C hrom atogr. 211 160-165
(10) SHI0N0, S ., KARIN0, I . , ISHIMURA, A ., EN0M0T0, J . (198o) J . C hrom atogr. 193 243-253
.-'ll) HAGNAUER, G .L ., SETTON, I . (1978) J . L iq u id C hrom atogr.
1, 55-73
12) HAGNAUER, G.L. (1980) Polymer C om posites 1 81-87
(13) C0UL0MBE, S ., SCHAUWECKER, P ., MARCHESSAULT, R .H ., H0UT- TEC0EUR, B. (1978) M acrom olecules 11_ 279-281
(14) BECK, W., HALASZ, I . and FRESENIUS Z. (1978) A nal. Chem.
291 312-318
(15) EISENBEISS, F ., DUMONT, E ., HENKE, H. (1978) Angew. m akro- m ol. Chemie 11_ 67-89
(16) KNOX, J . H . , MCLENNAN, F. (1979) J . C hrom atogr. 185 289-304 (17) ANDREWS, G .D ., VATVARS, A. (1981) M acrom olecules 14 1603-
-1605
(18) ZAB0RSKY, L.M. (1977) A n a ly t. Chem. 49 1166-1168
(19) HUDGINS, W.R., THEURER, K ., MARIANI, T. (1978) J . A p p l.
Polymer S e i. 34 145-155
32
(20) BRODILOVA, J ., ROTSCHOvX, J . , POSPI&IL, J . ( 1 9 7 9 ) J . Chro- m a to g r. 168 530-532
(21) KLEIN, J . , LEIDIGKEIT, G. (1979) Makromol. Chemie 180 2753-2756
(22) FLEER, G .J ., SCHEUTJENS, J.M.H.M. “A d so rp tio n o f i n t e r acting oligomers and polymers at an interface" Lecture at the Canberra conference, April 1981, to be pub
lished in J . Colloid and Interface Sei ., 1982
(23) SNYDER, L.R. (1968) P r i n c i p l e s o f A d so rp tio n Chrom atography M arcel D ekker, New York
(24) GLÖCKNER, G ., MEISSNER, R. (1980) A cta P o ly m erica 31
191-192 v
(25) BELENKIJ, B .G ., GANKINA, E .S ., TANNIKOV, M .B., VILENCIK, L .Z . (1976) Doklady Akademii Nauk SSR 231 1147-1149 (26) TERAMACHI, S., HASEGAWA, A., SHIMA, Y ., AKATSUKA, M .,
NAKAJIMA, M.' (1979) M acrom olecules 12 992-996 (27) DANIELEWICZ, M ., KUBIN, M. (1981) 9 5 1 - J S b
(23) BELENKIJ, B.G. (1979) Pure and A ppl. Chem. 51 1519-1535 (29) GLÖCKNER, G. (1980) J . Polymer S e i. C, Polym er Symposia
68 179-183
33
t