35(3$5$7,21$1',19(67,*$7,212)
3,//$5('/$<(5('&/$<6
3K'7+(6,6
3UHSDUHGE\
$1,.Ï6=$%Ï
(QYLURQPHQWDO6FLHQFH3K'6FKRRO
'HSDUWPHQWRI(DUWKDQG(QYLURQPHQWDO6FLHQFHV 8QLYHUVLW\RI9HV]SUpP
TABLE OF CONTENT
ABSTRACT... 4
KIVONAT ... 5
ZUSAMMENFASSUNG ... 6
INTRODUCTION ... 7
REVIEW OF THE LITERATURE ... 9
1. BASIC PRINCIPLES OF PILLARED CLAYS ... 9
1.1.THE VARIETY OF HOST MATERIALS...12
1.1.1. Fundamental aspects of smectites ...12
1.1.2. Characteristic features of Hungarian Bentonites ...17
1.2.THE VARIETY OF PILLARING SPECIES...18
1.2.1.Organic Cations...18
1.2.2.Organometal pillars...20
1.2.3.Metal Oxide Sols ...21
1.2.4.Metal Complexes...22
1.2.5.Polyoxycations ...23
1.2.6. Mixed Pillaring Species ...32
1.3.METHODS OF PREPARATION OF PILLARED CLAYS...34
1.3.1. The intercalation and exchange process...35
1.3.2. The washing and drying procedures ...35
1.3.3. The calcination and cross-linking mechanism ...35
EXPERIMENTAL PART... 38
2. PREPARATION OF PILLARED CLAYS ... 38
2.1.METHODS AND EQUIPMENTS FOR STRUCTURE ELUCIDATION OF PILLARED CLAYS...38
2.1.1. X-ray diffraction measurements...38
2.1.2. Elemental analysis ...40
2.1.3. Electron Microscopy ...41
2.1.4. Pore structure by adsorption-desorption techniques. ...41
2.1.5. FTIR spectroscopy. ...43
2.1.6. Thermal analysis ...43
2.1.7. Cyclic Voltammetry...44
2.2. SELECTION OF CLAYS...45
2.2.1. Morphology of Hungarian clays by Scanning Electron Microscope (SEM) pictures ...46
2.2.2. X-ray analysis ...51
2.2.3. Thermal Analysis ...60
2.2.4. Measurement of Cationic Exchange Capacity of host clays...61
2.3. SELECTION OF PILLARING SOLUTIONS...62
2.3.1. Aluminum – polyoxycations (Keggin-like ion) pillaring precursor ...62
2.3.2. Iron-oxide pillaring precursors...62
2.3.3. Organo pillaring precursor ...63
2.4. PREPARATION OF PILLARED SAMPLES...63
2.5. MAIN OBSERVATIONS FROM EXPERIENCES...64
RESULTS AND DISCUSSION 1. ... 66
3.1. ALUMINA PILLARED MONTMORILLONITES FROM HUNGARY ... 66
3.1.1. X-RAY ANALYSIS OF ALUMINA INTERCALATED AND PILLARED SAMPLES...66
3.1.2. CONTROLLING PORE DIMENSIONS...77
RESULTS AND DISCUSSION 2 ... 84
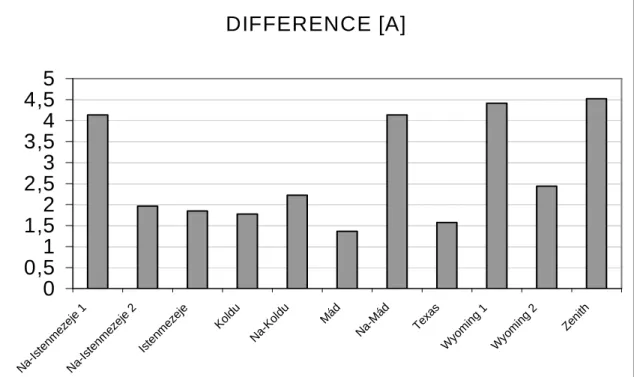
3.2. DIFFERENT KIND OF IRON-PILLARED MONTMORILLONITES... 84
3.2.1. PILLARING WITH IRON-CHLORIDE...84
3.2.2. IRON-BENZOATE AS A PILLARING AGENT...86
RESULTS AND DISCUSSION 3 ... 89
3.3. SILICA-PILLARED COMPOSITIONS ... 89
3.3.1.NATURE OF THE OLIGOMERIC ORGANOFUNCTIONAL SILOXANES. ...89
3.3.2.ION ADSORPTION...92
3.3.3.X-RAY ANALYSIS...94
3.3.4.INFRARED SPECTRA...98
3.3.5SURFACE AREA MEASUREMENTS...99
3.3.6.CYCLIC VOLTAMMETRY...101
SUMMARY ... 104
KEY – POINTS OF THE THESIS ... 112
REFERENCES ... 114
PUBLICATIONS ... 123
PRESENTATIONS... 124
ACKNOWLEDGEMENT ... 125
ABSTRACT
Pillared clays are artificial microporous materials created by pre-intercalation of layered silicates with robust polycations and subsequent thermal conversion of the polycations to nanoscopic metal oxide-like aggregates. The aim of the project was to produce and characterise three types of pillared clays.
Alumina-pillared clays were prepared by the intercalation of Hungarian bentonites with polyoxycations of Al3+. The resulting alumina-intercalated clays were heated at a lower temperature (350ºC) and also at a higher temperature (500ºC) to form the pillared forms. The structure of the intercalated and the pillared clays were evaluated using fundamental material characterization methods. The density of the pillars was calculated on the basis of chemical analysis. The conclusion reached was that alumina- pillared Hungarian bentonites could be used for industrial purposes.
Iron-oxide pillared clays were similarly prepared by intercalation using a Texas clay suspension (since the iron content of this clay is really very low) and various iron-poly- oxy-cations. While the intercalation process was successful, the heating resulted in the collapse of the microporous structure.
Silica-pillared clays were prepared by intercalation of a cubic octamer derived from the controlled hydrolysis of 3-aminopropyl-triethoxysilane using Greek smectite. The organosilicon cubanes were adsorbed in amounts exceeding the cation exchange capacity of the mineral. The excess quantity was incorporated into the form of physically adsorbed ion pairs, [(H3N-Pr)8O12Si8]. It was found that by varying the quantity of ion-paired silsesquioxanes in the clay galleries it was possible to control the free distance between the layers and thereby the surface area and the porosity of the resulting silica-pillared compositions. This demostrates the potential to create pillared- layered clays with tailor-made properties for industrial use.
KIVONAT
A pillérezett agyagásványok (pillared layered clays, PILCs) olyan mikropórusos
DQ\DJRNDPHO\HNUpWHJN|]LWHUpEHQQDJ\PpUHW IpP-polihidroxi-komplexekre cserélték
OH D W|OWpVNLHJ\HQOtW NDWLRQRNDW PDMG H]HQ NRPSOH[HNHW KHYtWpVVHO D UpWHJHNHW WDUWy
pillérként rögzítették. $ V]HU] DOXPtQLXP-oxid (Al-PILCs), vas-oxid (Fe-PILCs), és szilícium-oxid-pillérezett (Si-3,/&V PRQWPRULOORQLWRNDW iOOtWRWW HO W|EEIpOH EHQWRQLW pV W|EEIpOHSLOOpUH] NDWLRQVHJtWVpJpYHOPDMGMHOOHPH]WHV]HUNH]HWNHW
Al-PILCs
$V]HU] W|EEIpOHPDJ\DURUV]iJLEHQWRQLWHO IRUGXOiVEyOYHWWPRQWPRULOORQLWásványt pillérezett alumínium-polihidroxi-komplex (Al13-Keggin-ion) alkalmazásával. Az alumínium-polihidroxid-interkalált agyagásvány formákat egy alacsonyabb (350ºC)
LOOHWYHHJ\PDJDVDEEK PpUVpNOHWHn (500ºC) hevítve kapta meg a pillérezett formákat.
$ODSYHW DQ\DJV]HUNH]HWL PyGV]HUHN DONDOPD]iViYDO YL]VJiOWD D] HO iOOtWRWW LQWHUNDOiOW
és pillérezett formák szerkezetét. Kémiai analízis alkalmazásával számításokat végzett
D SLOOpU V U VpJUH YRQDWNR]yan. Megállapította, hogy az alumínium-oxid pillérezett magyarországi bentonitok ipari alkalmazása is lehetséges lenne.
Fe-PILCs
$ V]HU] YL]VJiOWD W|EEIpOH YDV-polioxi-KLGUR[L NRPSOH[V]HO W|UWpQ SLOOpUH]pV OHKHW VpJpW WH[DVL PRQWPRULOORQLW IHOKDV]QiOiVival, mivel az agyag eredeti vas tartalma rendkívül alacsony. Bemutatott sikeresen interkalált agyagokat, azonbanDSLOOpUH]HWWIRUPiNUHQGNLY OLQVWDELOQDNEL]RQ\XOWDN
Si-PILCs
$V]HU] HO iOOtWRWWV]LOtFLXP-oxid-pillérezett kompozitokat. A 3-aminopropil- trietoxi-szilán kontrolált hidrolízisével létrehozott kocka alakú oktamereket interkalálta=HQLWK DJ\DJKR] DQQDN NDWLRQFVHUpO NpSHVVpJpW PHJKDODGy DUiQ\EDQ pV tJ\
fizikailag adszorbeált ion-párok jöttek létre. Az ionpárok mennyiségének változtatásával befolyásolható a rétegek közötti bázis távolság, és ezáltal a szilícium-oxid pillérezett
NRPSR]LWIDMODJRVIHOOHWHYDODPLQWSRUR]tWiVDLV(]V]HPOpOWHWLDOHKHW VpJpWD]LSDUL DONDOPD]iVQDNPHJIHOHO WXODMGRQViJ~SLOOpUH]HWWDJ\DJRNHO iOOtWiViQDN
ZUSAMMENFASSUNG
Versäulte Tone („pillared clays“) sind künstliche mikroporöse Stoffe, hergestellt durch vorherige Interkalation von Schichtsilikaten mit starken Polykationen und anschließende thermische Umwandlung der Polykationen zu nanoskopischen metalloxidähnlichen Gebilden. Das Ziel des Vorhabens war die Herstellung und Beschreibung von drei Typen von versäulten Tonen.
Aluminiumoxid-versäulte Tone wurden durch die Interkalation von ungarischen Bentoniten mit Polyoxykationen von Al3+ dargestellt. Die so entstandenen, mit Aluminiumoxid zwischengeschichteten Tone wurden zur Bildung der versäulten Formen bei einer niedrigeren Temperatur (350ºC), und auch bei einer höheren Temperatur (500ºC) erhitzt. Die Strukturen der zwischengeschichteten und der versäulten Tone wurden unter Verwendung von grundlegenden Materialkennzeichnungsmethoden ermittelt. Die Dichte der Säulen wurde aus der chemischen Analyse berechnet. Die Schlußfolgerung war, daß die mit Aluminiumoxid versäulten ungarischen Bentonite für industrielle Anwendungen geeignet zu sein scheinen.
Eisen-versäulte Tone wurden auf ähnliche Weise durch Interkalation einer Tonsuspension aus Texas (der Eisengehalt dieses Tones ist sehr niedrig) mit verschiedenen Eisen-Polyoxykationen hergestellt. Zwar verlief der Interkalationsprozeß erfolgreich, doch ist das mikroporöse Gefüge beim Erhitzen zusammengebrochen.
Silika-versäulte Tone wurden durch die Interkalation eines kubischen Oktamers, hergestellt durch die kontrollierte Hydrolyse von 3-Aminopropyl-triäthoxysilan –unter Verwendung von griechischem Smektit – gewonnen. Die siliziumorganischen Cubane wurden in Mengen adsorbiert, die Kationaustauschkapazität des Minerals überschritten hat. Die Überschußmengen wurden in physikalisch adsorbierte Ionenpaaren [(H3N- Pr)8O12Si8] eingebaut. Dabei wurde gefunden, daß durch die Mengenvariation der Silsesquioxan-Ionenpaare in den Tongalerien der freie Abstand zwischen den Schichten und damit die spezifische Oberfläche und die der resultierenden siziliumversäulten Zusammensetzungen gezielt beeinflußt werden könen. Das deutet auf die Möglichkeit hin, versäulte-geschichtete Tone mit maßgeschneiderten Eigenschaften für industrielle Anwendungen bauen zu können.
Introduction
Clays such as beidellite and montmorillonite have layer structures. The layers are constructed of vertex and edge-sharing octahedra and tetrahedra. The atoms forming the layers are normally silicon and aluminum together with small mono- and divalent species such as magnesium and lithium. This framework layer has an overall negative charge which is balanced by the incorporation of cations, typically alkali metals, between the layers. It has long been known that these inter-layer cations can be readily ion-exchanged.
In the pillaring of clays the complexes to be exchanged into the inter-lamellar region are selected for size. Pillaring complexes (mainly inorganic cations as polynuclear hydroxy- metal type) may replace the alkali metals. Once a pillaring ion has been incorporated at a certain place between the layers, heating the modified clay results in dehydration and the linking of the ion to the layers. The resulting pillars support the sheets and the galleries thus creating a two-dimensional network of micropores, known as pillared layered clays. Because of the space created these kinds of structures are suitable for a wide variety of applications, e.g. adsorption, catalytic reactions.
Recent research into pillaring has been focussed in two main directions:
• Investigating a wider range or suitable host materials, e.g. metal phosphates,
• Investigating and extending the range of cations to be used in the cation replacement.
At first, the main obstacle to the production of economical pillared layered clays was the fact that they could only be prepared using pillaring suspensions with a very low concentration (less than 1% mass). As recent literature indicates, however, this problem has been overcome, and new processes have been developed which use pillaring suspensions with much higher concentrations.
The aim of the present research was to delevop and investigate several techniques for the incorporation of robust polycations in the different kinds of clays structure in order to produce cheap and useful new porous materials. Three combinations of host materials and polyoxycations were examined.
Particular emphasis was put on investigating bentonite rocks. There are large deposits of these rocks in Hungary, and the montmorillonite minerals, which they contain, have high cationic exchange capacity. This favorable combination of factors suggested that this bentonite rock could prove to be an economical source for artificially produced pillared layered clays.
Review of the literature
1. Basic principles of pillared clays
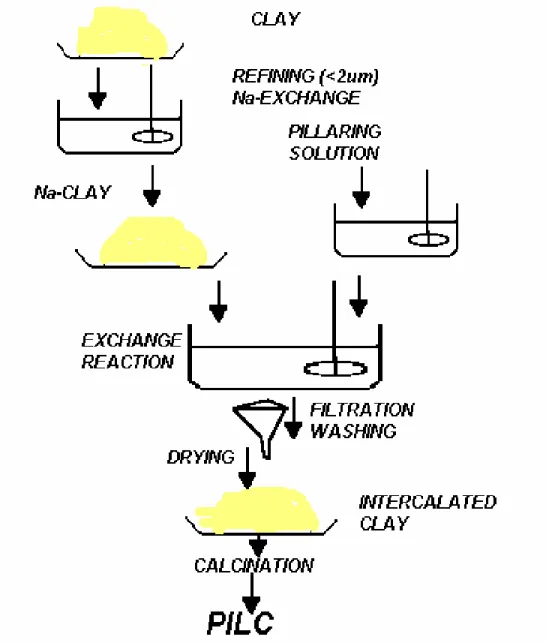
Pillared interlayered clays (PILCs) or pillared clays have received widespread interest as a novel class of microporous solid acids. PILCs are two-dimensional layer materials obtained by exchanging the charge-compensating cations (e.g. Na+, K+ and Ca2+) between the swelling phyllosilicate clay layers for larger inorganic hydroxy cations, which are polymeric or oligomeric hydroxy metal cations formed by hydrolysis of metal salts. Upon heating, the metal hydroxy cations undergo dehydration and dehydroxylation, forming stable metal oxide clusters which act as pillars keeping the silicate layers separated and creating interlayer spacing (gallery spaces) of molecular dimensions. The term of the intercalated clay or intercalation clay indicates the exchanged clay, while the term of the pillared clay defines the calcined product.
Figure 1.1. shows the representation of the differences in hydration-dehydration characteristics of smectite type clays and pillared clays. Thus, among the pillars and between the silicate lamellas a bidimensional network of zeolite-like cavities is formed.
Compared to zeolites, PILCs possess the advantage that by varying the size and separation of the pillars as well as their composition one may tailor the new materials to particular applications.
Previously, Barrer and his coworkers demonstrated that a series of molecular sieves and sorbants could be derived from smectites by exchanging their interlayer cations with alkylammonium or tetra-alkylammonium cations [1,2]. However, props of organic nature suffer from thermal instability since heating above 250ºC becomes catastrophic for the organic skeleton and in extension for the porosity of the material.
Much interest and research have, since 1970s, been directed toward the synthesis of pillared clays with higher thermal and hydrothermal stabilities. For these reasons later developments in the field were focused on the use of partially hydrolyzed metal cations as pillaring candidates. Thermal treatment of the intercalated oligomeric cations leads, following their dehydration and dehydroxylation, to metal oxide pillars characterized by enhanced thermal stability up to about 650ºC, large surface areas ranging between 200-350m2/g, sufficient acid sites and pores with sizes in the 1-10nm range. Any metal
oxide or salt that forms polynuclear species upon hydrolysis can be inserted as pillars, and all layered clays of the abundant phyllosilicate family as well as other layered clays can be used as the hosts [3-10].
Figure 1.1. Representation of the differences in hydration-dehydration characteristic of smectite type clays and pillared clays.
In order to obtain a good pillared product, some typical properties of the clays and the pillars are required: (a) the clay should have a moderate CEC and the pillaring cation a high positive charge; (b) the clay should swell in polar solvents and the pillaring cations should dissolve in this solvent; (c) the interlamellar cations should exchange easily and the clay sheets must have the appropriate size to be stable enough after the intercalation; (d) it seems that Na+ montmorillonite (2 µm fraction) is a good starting material for the following reasons:
1. The monovalent Na+ exchanges well with most other cation, especially if the pillaring species have a higher charge.
2. Na+ hydrates well and makes the clay layers swell.
3. Na+-refined montmorillonite is easy to prepare (exchange with NaCl) and inexpensive.
4. Na+ is not too small and remains exchangeable 5. Montmorillonite has an intermediate CEC.
6. The 2 µm fraction is commercially available (inexpensive) since this fraction is used often for its rheological properties in industry.
7. Purification of natural montmorillonite by centrifugation results in the pure 2µm fraction, so this powder contains very little impurities.
8. 2µm particles are small enough to be homogeneously intercalated and large enough to be rigid and stable.
9. Smaller fractions result in a randomly stacked powder (face-to-edge, FE tactoids).
10.The montmorillonite particles do swell in water, but do not delaminate in suspension.
They therefore keep a more or less ordered face-to-face (FF) stacking.
Various undermentioned chemical and crystallographic parameters of the clay have all been shown to influence the physical and chemical properties of pillared clays [11-13]:
(1) the magnitude of the layer charge, (2) the location of the charge, (3) the distribution of the charge, (4) and the nature of the octahedral sheet.
The following experimental parameters can all affect the degree of polymerization of the hydroxy-oligomeric cations in aqueous solution, consequently the physicochemical properties of the pillared clays [14-15]: (1) concentration of the metal ions, (2) basicity or degree of hydrolysis (given as r = OH/M), (3) temperature of preparation, (4) time and temperature of aging, (5) type of counterion, (6) and the method of preparation.
In the following some applications of PILCs under investigation will be reviewed: (1) ion exchangers and ion-selective membranes, (2) ultrafiltration and membranes, (3) selective adsorption of gases, (4) pollutant scavenging and waste management:, (5) sensors, (6) acid catalysed organic reactions, (7) non-acid catalysed organic reaction, (8) photochemistry and photocatalysis, (9) immobilization of enzymes, (10) electrochemically active coatings, (11) protective coatings.
1.1. The variety of host materials
As soon as it became clear that pillaring clays was an easy and controllable way to introduce specific micropores in a rather inexpensive material, also other layered structures were used for intercalation. Examples are: layered double hydroxides [16], metal phosphates and metal phosphonates [17-18], and layered silicic acids such as magadiite [19], titanates [20-23], manganese titanates [24], and niobates [25]. The first microporous pillared solid having semi-conducting properties with high surface area and high thermal stability was prepared by manganese titanate.
In this project Hungarian bentonites and a few other kinds of montmorillonites were applied as host materials, thus in the following a short review of fundamental aspects of smectites, and the characteristic features of Hungarian bentonites will be given.
1.1.1. Fundamental aspects of smectites
Natural clays are the products of weathering of rocks [26]. From the structural point of view, all clays are layered silicates, also described as phyllosilicates (phyllo = leaf-like).
Table 1.1. shows the classification of natural and synthetic smectites. Dioctahedral aluminous smectites are represented by the montmorillonite-beidellite series according to the structural formula
(Al
2-yMg
y2+)(Si
4-xAl
x)O
10(OH)
2E
+x+y.nH
2O .
Where the amount of E+ represents the interlayer cation,
x
andy
the octahedral and tetrahedral substitutions, respectively. The smectites withy>x
are called montmorillonite (Figure 1.2.), and those withy<x
are called beidellites. Appreciable amounts of trivalent Fe often occur in octahedra. Montmorillonites are commonly the main constituents of the rocks known as bentonites, whereas beidellites are frequently found in soils as weathering products of detrital micas.The basic structural components of the smectites are the octahedral (consists of two planes of spherical anions (O, OH)) and tetrahedral sheets (are composed of six-fold hexagonal rings) and interlayer configurations.
The final structure of a clay sheet is the result of a condensation of the tetrahedral silica sheets with the octahedral sheets. This happens by sharing the apical oxygens of the silica layer with the free oxygens of the octahedral layer (Figure 1.3.).
Table 1.1. Classification of natural and synthetic smectites [26].
DIOCTAHEDRAL SMECTITES TRIOCTAHEDRAL
SMECTITES Ratio between Predominant Smectite Predominant Smectite Tetrahedral (xt)
and
Octahedral Species Octahedral Species
Octahedral (x0) Cation (s) Cation (s)
Charges
X0/xt > 1.0 Al(R2+)* Montmorillonite Mg Stevensite
(octahedral Mg(Li)* Hectorite
Charges AlMgLi Swinefordite
Predominant)
Single Transition
Mixed Metal
Transition "defect" trioc.
Metals Smectites
Xt//x0 > 1.0 Al Beidellite Mg Saponite
(tetrahedral Fe3+ Nontronite Fe2+ Iron saponite
Charges Cr3+ Volkonskoite Zn Sauconite
Predominant) V3+ Vanadium Co Cobalt smectite
Smectite Mn Manganese
smectite Single or Transition
Mixed Metal trioc.
Transition Smectites Metals
Both octahedral and tetrahedral cations might be substituted by other elements, as long as these new cations have the appropriate size to fit in the structure (e.g.
Si
4+oAl
3+,Al
3+oMg
2+,Mg
2+oLi
+). The phenomenon is called ’’isomorphic substitution’’ and is responsible for some very important properties of the clay minerals [27]. Since the substituting ions might have another charge (mostly a lower valence), the initially neutral clay sheet will now carry a net negative charge. This excess of negative layer charge is compensated by the adsorption on the layer surfaces of cations, which are too large to be accommodated in the interior of the crystal.In the presence of water, the compensating cations on the layer surfaces may be easily exchanged by other cations when available in solution; hence they are called
“exchangeable cations”. The total amount of these cations may be determined analytically. This amount, expressed in milliequivalents per 100g (or per gram) of dry clay, is called the cation exchange capacity (CEC) of the clay. One equivalent of Ca2+
would be equal to one mole divided by the oxidation state of calcium or could be a combining weight of 40 divided by the oxidation state. The number of interlayer cations is assumed to be stoichiometric with respect to the net negative layer charge. Although cations with higher valencies may substitute in the interlayer, the univalent and divalent cations are more common.
Figure 1.2. Diagrammatic sketch of the structure of the montmorillonite after Grim [28].
The property of ion exchange is great fundamental and practical importance in the investigation of the clay minerals. In the application of clay mineralogy it is important because the nature of the exchangeable ion may influence substantially the physical properties of the material. Thus, a clay material carrying sodium frequently has very different plastic properties than material the same in every way except that calcium is the exchangeable cation. There are various methods to determine the CEC [29-39], however, they can give mutual differences. This is because the charge on the sheets does not only arise from the isomorphic lattice substitution, there is also a contribution from broken bonds at the edges of the layers, and from protonated or de-protonated hydroxy-groups.
Figure 1.3. The condensation of a tetrahedral and octahedral layer results in the formation of so-called TO-clay sheet after Nemecz [27].
As a result, there is a pH-independent part of the CEC, which arises only from the isomorphic substitution, and a pH-dependent part. The pH-dependent CEC might be up to 10% of the total CEC and depends strongly on the crystal size and shape, pH, type of exchangeable cation and also the method that was used to determine the CEC.
It has generally considered that the CEC of montmorillonite does not change substantially. The change in CEC is reduced on heating to various temperatures but the effect is not uniform and varies with the cation present.
Another noticeable property is the mobility of the interlayer cations in the clay structure.
Upon heating the clay, these cations might irreversibly migrate into the octahedral layer of the clay. The effect is called the Hofmann-Klemen effect [40] and four conditions have to be fulfilled in order to see this migration (Figure 1.4):
• The clay layer must have a negative charge, from the octahedral layer.
• The octahedral layer must have vacant sites (as in dioctahedral clays).
• The cations should be small enough to migrate through the Si-O 6-rings of the tetrahedral layer into the octahedral part of the clay sheet.
• The effect occurs mainly at increased temperatures.
Figure 1.4. The Hofmann-Klemen effect. Here the migrations of Li+ ions through the ditrigonal Si-O rings of the T layer into the vacant cation sites of the octahedral layer.
The result of the Hofmann-Klemen effect is that the interlamellar cations are trapped in the octahedral layer and cannot be exchanged for other cations any more. Not only looses the clay its CEC, also its ability to swell in polar solvents is lost.
The HK-effect might be used however in a positive way, namely to reduce the charge or CEC of clays in a controlled way or to distinguish dioctahedral clays with isomorphic substitution in the octahedral layer from those with substitution in the tetrahedral layer.
If the Li+-form of the first type is heated, then the clay will not re-expand any more due to the HK-migration of the lithium ions. In the latter case, the negative charge is located in the tetrahedral part, and Li+ can not migrate into the dense tetrahedral structure.
Since the cation remains in the interlamellar space, even after heating, this type of clay will still be able to expand in polar solvents. For some applications however, it might be useful to select other clay minerals. If, for some reason, the HK-effect has to be
avoided, one has to choose a trioctahedral smectite, saponite [41]. Rectorites are used if high thermal stabilities are required [42] and for the investigation of the alumina pillars with 27Al-MAS-NMR, it is better to choose an Al-deficient clay, hectorite [43].
1.1.2. Characteristic features of Hungarian Bentonites
Clay minerals can form in a lake whenever the lake contains starting materials (minerals) capable of hydrolysis. Hungarian bentonites are the hydrolysis products of feldspars and volcanic glass fallen into water, but only a smaller percentage can be considered lacustrine: a major part is regarded as marine [26].
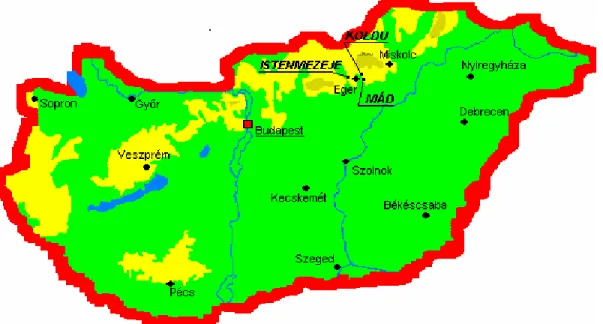
Figure 1.5. Schematic map of Hungary with the bentonite regions of Istenmezeje, Koldu and Mád
Since in absorption from sea water, the law of mass action is replaced by ionic intensities as the prime controlling factor, Mg and Ca ions are mostly absorbed to the detriment of Na, which leads to the assumption that bentonites largely containing alkali earths are of marine origin. The much scarcer original Na-bentonites were presumably formed in lakes poor in Ca and Mg and rich in Na ions. The predominant Hungarian bentonites are with Ca as the leading exchangeable cation. In this work Hungarian
bentonites were taken from the north part of Hungary, namely such as Istenmezeje, Koldu and Mád. The places of these villages can be seen on Figure 1.5.
At the above-mentioned mines, bentonites are found in large quantities, therefore they might have been cheap raw materials of pillared layered structure for certain industrial applications.
1.2.The Variety of Pillaring Species
Besides the variety in host materials, there are also a number of pillars that can be intercalated [10]. Factors that are important for the choice of a certain pillaring specie are: a) thermal and hydrothermal stability of the final PILC, b) interlayer and interpillar distance, c) chemical nature of the pillars and their catalytic activity, and d) chemical stability of the pillars. Depending on the final purpose of the PILC, one might choose the appropriate pillars. This flexibility is one of the main advantages of pillared clays!
In the following the practicable pillaring species will be reviewed, namely such as (1) organic cations, (2) organometal pillars, (3) metal oxide sols, (4) metal complexes, (5) polyoxycations, and also (6) mixed species. In this research work, organic cations (aminofunctional silanes) and aluminium-, iron-polyoxycations were applied as pillaring species.
1.2.1.Organic Cations
The adsorption of organic molecules by clays has been known already for a long time but the first attempts to expand layered silicates permanently and introduce interlamellar micropores were performed by Barrer [1]. By using alkylammonium and alkyldiammonium ions with various chain lengths, interlayer distances were obtained between 2.2 and 4.0 Å. The surface area of these organo PILCs went up to 300 m2/g (CH3NH3+
) and micropore volumes of 0.098 cm3 / g were obtained (+NH3(CH2)4NH3+
).
The research on amine intercalated clays was very lively and especially the use of the more stable bicycloamines (e.g. DABCO or 1,4-diazobicyclo(2,2,2)-octane) caught
much attention [44]. Intercalated smectites with these diamines are sometimes called crosslinked organo smectites (CLOS).
The use of functionalized silicon reagents represents a clever approach for the synthesis of silicon pillared clays [9, 45]. Aminofunctional silanes of the type X3Si(CH2)nZ, where X represents an easily hydrolyzed group e.g. –OCH3, -OC2H5 or – Cl, Z is a pyridyl group for n=2 or –NH2, -NR2 and NH(CH2)2NH2 for n=3, are excellent precursors for the synthesis of silica pillared clays [46-47]. Under controlled conditions of hydrolytic condensation these pillaring precursors are converted to a family of there dimensional organosilicon oligomers composed of a polyhedral silicon-oxygen skeleton with intermittent siloxane chains that bear the amino functionalized subtituent [46].
The various amino functionalized oligomeric derivatives can not be isolated but gel permeation chromatography for the Z=-Net2 member [48], 29Si NMR spectroscopic studies for Z=-NH2 member [49], have shown that the respective octameric species II are predominantly formed in methanolic or ethanolic solution of the monomers upon addition of water. The cubic silsesquioxanes present certain special features, which make them ideal pillaring agents for interactions with smectite clays. First, protonation of the amino groups gemnerates oligomeric cationic species with expendable and bulky organic groups that can be easily inserted into the lamellar zone of the clay and then can be removed by thermal treatment leaving behind silica pillared structures. Second, the cubic geometry of the various amino-members offers only one possible orientation for placement in the interlayer zone of the clay. Third, the various aminofunctional silanes possess the ability to bind metal ions (Mn+) yielding metal complexes, [X3Si(CH2)nZ]xMn+ , that are the same as those derived from the related organic ligands without the X3Si group. This important property makes possible the design and synthesis of metal complexes in which the metals center is coordinated to the functionalized group of the siloxane octamer. The resulting cationic complexes are expected to be excellent pillaring precursors for the synthesis of metal-substituted silica pillared clays.
These important properties and also the lack of systematic studies describing the interactions of clays with cage-like organosilicate materials prompted us to study the intercalation of sodium montmorillonite with a typical octameric silsesquioxane derived
from 3-aminopropyltriethoxysilane in this research work, in detail. Particular emphasis was given to the factors that affect the nature of the intercalated products and the pillared structures obtained by subsequent thermal processing.
1.2.2.Organometal pillars
Other pillaring agents were used to increase the thermal stability and, in the mean time, to introduce catalytic active elements in the microporous pillared clay. A good attempt was made by using ionic metal chelates, such as tris(ethylene-diamine)cobalt(III) or briefly Co(en)33+
and metal(2,2-bipyridine) or metal(o-phenanthroline) complexes [50- 52]. Intercalated Rh(PPh3)2
+-hectorite was used for the selective hydrogenation of 1- hexene. Since the reaction results in the formation of a mono- and dihydride intermediate of the pillars, the neutral Rh-complex might desorb from between the hectorite layers.
Rh(PPh3)2+
+ H2 Æ RhH2(PPh3)2+
<-> RhH(PPh3)2 + H+ (1.2.-1)
To avoid this problem, some ligands were replaced for inactive, positively charged species, namely phosphonium-phosphine ions [(C6H5)2P(CH2)2P(C6H5)2(CH2C6H5)+ or (P-P)+]. As a result, the catalytic active organo rhodium pillars RhH2Cl(P-P)x
+ stay positively charged during the reaction and remain between the clay sheets. In fact, these systems are an intermediate between a real pillared clay and a catalyst, intercalated between the clay layers. They were not synthesized in the first place to introduce permanent porosity but because the yield of a selected catalyzed reaction was higher if the catalyst complex was between the clay layers instead of in solution.
Other examples of these systems are Rh(NBD)(dppe)+-hectorite (NBD=norbornadiene;
dppe=1,2-bis(diphenylphosphino)ethane), RhH(CO)x(P-P)2+
and Rh[(R)-4-Me- Prophos]+-montmorillonite (where the ligand is an asymmetric bidentate of (CH3C6H4)2PCH(CH3)CH2P(C6H4CH3)2. The latter system yields 95 % optical pure chiral precursors of the famous drug L-Dope [10].
The advantages of organo-metal pillared clays are the good intercalation of the complex and the related catalytic activity. Despite these facts, they remain thermally
unstable due to the presence of C-C-bonds (decomposition at 350-450°C), exchangeable and do not tightly connect the adjacent clay layers together, so that swelling still occurs.
Another approach to form pillared clay with interlayer organo-metals was proposed by Endo [53-54]. Here, an organo-metal complex precursor (e.g. Si(acac)3
+ or tris(acetylacetonato)silicon(IV), is adsorbed between the clay sheets and converted into the silicic acid form by a controlled, in situ hydrolysis.
[Mn+] + Si(acac)3
+ Æ Mn+ + [Si(acac)3
+] (1.2.-2)
[Si(acac)3
+] + 4H2O Æ [Si(OH)4 + H+] + 3H(acac) (1.2.-3) (The bold/italic formulas between brackets represent molecules between the clay sheets)
After oxidation at 500ºC, a silica residue remains between the clay layers. The final product has an interlayer distance of 3 Å and a BET surface area of about 200 m2/g.
Another typical organo-metal pillar is the trinuclear Fe(III)acetato complex cation. This complex is prepared by the reaction of Fe(III)nitrate and acetic acid in acetone [56-60].
The exchanged complex is converted into iron oxide pillars upon heating at 350 C. It seems however that the intercalation is hard to control and difficult to reproduce, since many authors find different interlayer distances. It is shown by Maes et al. [60] that the Fe(III) acetato complex is partially hydrolyzed between the clay layers. As a result, the intercalated pillaring species change in size. Basal spacings between 6 and 10 Å are found and the resulting surface area of the Fe-PILC is between 100 and 300 m2/g and seems to remain till 500ºC.
1.2.3.Metal Oxide Sols
Pillared clays were prepared by the direct intercalation of metal oxide sols (abbreviated as DIMOS) [61]. Aqueous oxide sols with a particle size between 20 and 80 Å may be intercalated between the clay sheets and form so-called supergallery PILCs, a term that indicates all substrates in which the interlayer distance is substantially larger than the thickness of the host layers. Sols of uniform particle sizes and carrying a positive
charge are required and good results were obtained with silica and alumina particles [62].
Spectacular intercalations with tubular aluminosilicates (imogolite) resulted in interlayer distances of ± 34 Å. These Tubular Silicate Layered Silicate (TSLS) nanocomposites have a thermal stability up to 450ºC and a BET surface area of 460 m2/g. Micropore space is available in both intra- and intertubular channels. Mixed SiO2-TiO2 sol particles were prepared by Yamanaka [63]. This procedure of intercalation shows that there is no straight line in the subdivision of the pillaring particles. The silica-titania particles for instance, are intercalated as organometal complexes, present in a sol solution and converted into oxide species after calcination. Sol particles are prepared by hydrolysis of Si(OC2H5)4 and Ti(OC3H7)4 with HCl and exchanged with the interlayer cations of the clay. The PILC is stable till 500ºC and has a surface area of about 400 m2/g and an interlayer distance between 25 and 35 Å. From nitrogen adsorption isotherms and analytical experiments, it was found however that the pores in the PILC were smaller than the interlayer distance. Therefore, it was proposed that not the individual particles form the pillars, but that the layers are expanded by packed clusters of SiO2 particles with TiO2 on their surface. It is worth mentioning that these SiO2-TiO2 PILCs were also prepared by a controlled rearrangement of the sol particles.
By intercalating simultaneously the oxide sols and an organic template (octadecyltrimethylammonium ions or OTMA) and burning off the organic molecule afterwards, a PILC was obtained with an interlayer spacing between 30 and 40 Å, a surface area of 600 m2/g and a pore volume of about 0.8 cm3/g.
1.2.4.Metal Complexes
The use of metal cluster complexes was also proposed to introduce mainly transition metals and their oxides between the clay layers [64]. Niobium, tantalum and molybdenum chlorides [(Nb6Cl12)n+, (Ta6Cl12)n+ and (Mo8Cl8)4+] were exchanged with the interlamellar sodium ions and oxidized at 240ºC under vacuum to form the metal- oxide clusters. The reaction involves ion exchange, hydrolysis and oxidation with water as the oxidizing agent:
(M6Cl12)(H2O)6
n+ + [nNa+] Æ [(M6Cl12)(H2O)6
n+] + nNa+(aq.) (1.2.-4)
[(M6Cl12)(H2O)6n+
] + H2O Æ [(M6Cl12)(OH)x(H2O)6-x](n-x)+ + H3O+] (1.2.-5)
The hydrolytic equilibrium shifts to the right as the cluster loading increases, and the resulting H3O+ is displaced by more metal clusters until a complete layer of hydrated cations covers the interlayer surface. Oxidations at elevated temperatures might be represented as:
[(M6Cl12)(OH)(H2O)5
+] + 9H2O Æ [5(M2O5) + H+] + 12HCl + 8H2 (1.2.-6)
Interlayer distances of 10 Å and a surface area of 64 m2/g were observed and the thermal stability of this oxide cluster intercalated product is about 400ºC.
A Sn-PILC was prepared by the intercalation of (NH4)2SnCl6 in montmorillonite [65].
The Sn-PILC had an interlayer spacing of ± 5 Å.
1.2.5.Polyoxycations
Polyoxycations of various metals are by far the most popular pillaring species that are described in PILC literature. Their success should be attributed to the following properties: (1) most of them are easy to prepare in a reproducible way; (2) they carry a positive charge; (3) they exchange well with the Na+ ions between the clay sheets; (4) they are readily converted by heating into very stable metal oxide pillars; (5) they tightly connect adjacent clay layers to each other; (6) they are large and give good interlayer distances.
First of all, alumina- and iron-pillaring species will be presented in detail, because this research work was focused these kind of polyoxycations. Subsequently, a review about the other kind of polyoxycations will be given.
1.2.5.1. The pillaring precursor [Al13]7+
The intercalation of clays with polyoxycations of Al3+ was not only one of the first inorganic PILCs, it is also the most investigated PILC till now. The main pillaring agent that is responsible for the 10 Å interlayer distance in alumina pillared clays, is the [AlO4Al12(OH)24+x(H2O)12-x](7-x)+ (briefly Al13).
Figure 1.6. Line Drawing of the [Al13O4(OH)24(H2O)12]7+ Keggin ion
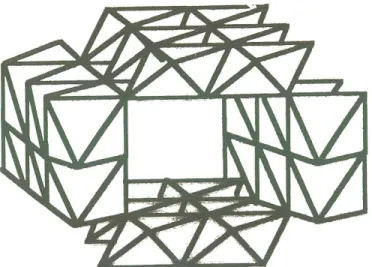
This polyoxycations is the result of a controlled hydrolysis of Al3+ salts. The pH measurements give direct information on the hydrolysis process. The structure of aluminum-polyoxycations was characterized by several methods [66], namely such as NMR, SAXS, Raman Spectroscopy, Light Scattering, Ultracentrifugation and Size Exclusion or Gel Permeation Chromatography. From all these data, the Al13-oligomer was described as a Keggin ion [67, 68], which has the shape of a prolate spheroid, that
consists of one central AlO4 tetrahedron, surrounded by twelve octahedra of aluminum hydroxide (Figure 1.6.). The three-edge-sharing octahedra have free corners that are occupied by oxygen, bridging hydroxyl groups or bridging water molecules. Each octahedral Al3+ ion is at equal distance from its neighbors. The ion is rather symmetrical and contains all symmetry elements of a tetrahedron. Along its diagonal axis, the Al13-oligomer is 8.8 Å from the upper to the lower oxygen. If OH groups are present, then 0.6 Å is added for each O - H bond length and an approximate value of 10 Å is obtained. The largest direction is found along the C2 axis (10.3 Å or 11.5 Å if OH groups are assumed). The tetrahedral structure is revealed in the macrostructure of this Keggin ion crystal [69]. Figure 1.7. shows the SEM picture of Al13-crystals.
Although the ideal formula represents a charge of 7+, it may be changed upon hydrolysis of the ion. By modeling the deprotonation reaction with alkalimetric titration data, it was found that the hydrolysis reaction could best be described as a sequence of six two-deprotonation steps. This phenomenon occurs also if the Keggin ions are intercalated between the clay layers [70, 71].
Depending on the preparation procedure, one finds various aluminum monomers or polymers in the solution. In general, five methods are used to prepare hydrolyzed [Al13O4(OH)24(H2O)12]7+ ions [72]: (1) Hydrolysis of aqueous Al3+ salt solutions (most often AlCl3) with NaOH. (2) Hydrolysis of aqueous Al3+ salt solutions (most often AlCl3) with Na2CO3 (the carbonate anion reacts with H3O+ and forms OH- groups and CO2 gas). (3) Electrolysis of AlCl3. (4) Dissolution of Al metal in HCl (with liberation of H2). (5) Dissolution of Al metal in aqueous Al3+ salt solutions (with liberation of H2). In this research work, AlCl3 solution was hydrolyzed with NaOH.
The composition of the hydrolyzed Al3+ solution depends strongly on the preparation conditions. Several species might be present besides the Al13-ion: monomers of Al(H2O)6
3+, dimers Al2(OH)2
4+, trimers Al3(OH)4
5+, octamers Al8(OH)20
4+ and polymeric species of [Al(OH)3]n, sometimes described as ring polymers from the basic ring unit [Al6(H2O)12(OH)12]6+ or from the polymerization of Al13 entities [73, 74].
Figure 1.7. SEM photograph of Al13-crystals after Molinard [72]
Figure 1.8. Speciation of 10-2M aluminum for [OH] / [Al] up to 2.5 after Bottero et al.
[73,74]
The controlled hydrolysis of AlCl3 solution with NaOH in the range 1.5 < [OH] / [Al] <
2.3 should result in a solution with Al13-oligomers as the main species (Figure 1.8.).
The exact composition and distribution of the species in solution however, depends on the Al3+ concentration, the temperature and the hydrothermal treatment, the age of the solution and the aging temperature, the source of Al3+ and the preparation method.
Since the charge of the Keggin ion depends on the degree of hydrolysis and therefore the pH, it is possible to charge this value in a controlled way. Evidence for this charge variation was found by Vaughen [75]. Aluminum(III) hydrolysis and precipitation in the presence of acetic acid and oxalic acid have been studied by combining potentiometric titration and liquid-state 27Al NMR [76]. Different aluminum species have thus been identified and followed according to the pH and the L / M (ligand to metal) ratio:
unreacted and hydrolyzed monomers, complexed forms, and the Al13 tridecamers were directry recorded by NMR, and a “solid” species, not detected by liquid-state NMR, has been determined by difference. The level of perturbation of aluminum hydrolysis by organic acids can be related to the strength of complexation. Thus, the speciation depends on differential acid-base affinity between aluminum and carboxylate, and aluminum and hydroxyl. The nature of the “solid” species is hypotetical. 27Al NMR studies of aluminum clusters have one major drawback [77]. At lower field strengths, some aluminum nuclei may not be detected. Al nuclei having large quadrupole coupling constants (QCC), as a result of low coordination symmetries, can give signals broadened beyond detection [78]. Solid state 27Al NMR detects nuclei with high QCCs provided that sufficiently large magnetic field strengths are used. In research work of Parker et al. [77] the species of Al13 tridecamer were examined by performing 27Al NMR on the solutions during aging and by studying the precipitated sulfate salts via solid state 27Al NMR and powder XRD. There was no evidence of M incorporation; only Al13
was found. The XRD patterns are indexable in the cubic F¯ 43m space group. In fact, different polymorphic structures of sulfate salt can be obtained, depending on solution pH.
The pillared clays obtained with the Keggin-ion exhibit Brönsted or Lewis acidity, or both, depending on the nature of the clay. This acidity is believed to be a consequence of the dehydration / dehydroxylation reaction that occurs upon calcination of the intercalated polycation at elevated temperatures [79]:
[Al13O4(OH)24(H2O)12]7+ 6.5 Al2O3 + 7 H+ + 20.5 H2O (1.2.-7)
The physical properties of pillared clays that most significantly affect their catalytic behavior are porosity and acidity.
1.2.5.2. The iron pillaring precursors
The aqueous chemistry of Fe(III) is known to yield polymeric cations [80-84] of substantial size and there have been reported attempts to intercalate such ions in the interlayers of smectite clays [85-88]. The characterized crystalline iron(III) oxides and hydrous oxides are Fe2O3 and FeO(OH), each of which is polymorphic. The phases
LPSRUWDQW LQ WKH K\GURO\VLV DQG SUHFLSLWDWLRQ RI )H,,,VDOWVDUH - FeO(OH), goethite;
-)H22+DNDJDQHLWHDQG -)H22+OHSLGRFURFLWHDVZHOODV -Fe2O3, hematite.
The iron hydrous oxides are built of double chains of edge-shared Fe(O,OH)6
octahedra (Figure 1.9.). In contrast, the structure of Fe2O3 does not contain this double chain element. In aqueous Fe(III) salt solutions the following species exist: Fe3+, Fe(OH)2+, Fe(OH)2
+, Fe(OH)2
4+, and Fe2O4+. A survey of Fe(III) coordination in oxides, hydroxides, and aqua species shows that Fe(III) in acidic oxide environments, including aqueous solutions, is octahedrally coordinated [89]. Only in basic environments does Fe(III) occur in tetrahedral coordination to a significant extent;
example include Na5FeO4. The hydrolysis of inorganic Fe(III) solutions consists of several steps: (1) formation of low-molecular-weight species; (2) formation of a red cationic polymer; (3) aging of the polymer, with eventual conversion to oxide phases;
and (4) precipitation of oxide phases directly from low-molecular-weight precursors.
Figure 1.9. The schematic crystal structure of proposed akaganeite type of iron- pillaring complex.
Most of the hydrolysis studies have been conducted of solutions of Fe(III) nitrate, perchlorate, or chloride in the concentration range 10-3-10-1 M, at room temperature.
The behavior of hydrolyzed Fe(III) solutions depends on the nature and mode of addition of basic reagents. Ordinary mixing of solutions of alkali hydroxide or ammonia with Fe(III) solutions results Fe(III) in immediate formation of a precipitate; if the amount of base added corresponds to PROEDVHSHUPROHLURQWKHSUHFLSLWDWHUH- dissolves. For reliable characterization of the hydrolysis process, it is necessary to add base so that precipitation does not occur. At higher temperatures the aging processes are accelerated. The pH of hydrolyzed Fe(III) solutions is observed to decrease with time. Polymer produced in chloride solutions consists of spherical particles like those obtained in nitrate or perchlorate solutions. The course of pH changes on aging is accelerated by the presence of Cl¯ . Substitution of Cl¯ by OH¯ in the polymer may contribute to the accelerated pH decrease. The lifetime of the polymer decreases with increasing temperature, so that titrations conducted at 90ºC result in precipitation without formation of soluble polymer. The solids precipitated have studied during high- temperature hydrolysis without addition of base by XRD. In the presence of chloride,
DNDJDQHLWH - FeO(OH) is produced. The precipitates obtained by addition of bases to Fe(III) solutions under a wide variety of conditions are amorphous to X-rays and are not stoichiometric hydroxides. Aging of the amorphous gels requires years at room temperature, and hours to days at 100ºC; the product formed are goethite or hematite.
Polymerization of iron typically begins at low pH (<1.5) and propagates by deprotonation of coordinated water molecules (olation) and hydroxylgroups (oxolation) as illustrated in Eqs.(1.2.-8) and (1.2.-9):
[Fe(OH)2+
]n + Fe3+ + 2H2O Æ [Fe(OH)2+
]n+1 + 2H+ (1.2.-8)
[Fe(OH)2
+]n Æ [FeO(OH)]n + nH+ (1.2.-9)
The hydrolysis reactions of Fe(III) can lead to discrete spherical polycations as large as 30 Å in diameter. Aggregation of the spheres produces rods and eventually rafts of rods. The polymerization process is dependent on base to metal ratio, temperature, and nature of the counterion, pH, and other factors. Studies carried out on fully
hydrated precipitates using X-ray diffraction showed that the structural continuity exists [90-91]. Rightor et al. [92] found that the iron oxide pillared clays formed by the reaction of sodium montmorillonite with hydrolyzed Fe3+-solutions depended critically on the hydrolysis conditions. The basal spacings of iron oxide pillared clays (Fe-PILC) between 18 and 29 Å may be obtained by using iron(III)nitrate, chloride or perchlorate, hydrolyzed with sodium carbonate. Bradley and co-workers found [93] that Fe13-ion formed by hydrolysis of Fe(III) solution. It appeared that this Fe13-ion(7+) is extremely unstable and decomposes very rapidly in solution. The additional of small amounts of Fe(II) ions seems to stabilize the structure somewhat, and allowed it to be used as a clay mineral pillaring agent. These results suggest very strongly that analogous Fe13
and Ga13 species form upon the base hydrolyses of aqueous iron(III) and gallium(III) solutions.
1.2.5.3. Review of other kind of precursors
Some structures of the pillaring precursor cations are elucidated already, while others are still subject of discussion.
Ga
Some work has been done on Ga-PILCs [94,95]. The gallery heights are somewhat more than 10 Å at room temperature around 8 Å at 600ºC. At higher temperatures, a collapse of the PILC occurs. The surface area of this Ga-PILC drops from 230 m2/g at room temperature to 170 m2/g at 600ºC. It is worth noting that the pillaring precursor cations of the Ga-PILC are identical in structure to those of the alumina pillared clay, namely [GaO4Ga12(OH)24(H2O)12]7+. This PILC seems to have a very good catalytic performance.Zr
Small but stable interlayer species are formed by zirconium tetramers [Zr4(OH)8+x(H2O)16-x](8-x)+ [96]. Depending on the method of preparation, which is most often the hydrolysis of ZrOCl2 (zirconylchloride), these Zr-PILCs exhibit spacings between 3 and 14 Å and surface areas between 100 and 400 m2/g [97-100]. They might remain stable up to 700ºC [101].Ti
Very large basal spacings (almost 30 Å) on the other hand are obtained by the intercalation of TiO2 pillars. The final Ti-PILC is prepared by calcination of clay, intercalated with hydrolyzed TiCl4 or Ti-alkoxides [102-106]. The polymers are described as [TiO(OH)2]n and surface areas up to 350 m2/g are reported. The pore volume and surface area seems to remain constant to about 700ºC.Bi
Hydrolysis of bismuth perchlorate results in the formation of [Bi6(OH)16]2+polycations (Figure 1.10.). Bi-PILCs have interlayer spacings of about 6 Å and a surface are of approximately 80 m2/g. The porosity drops steadily upon heating the PILC at temperatures above 200ºC [107].
Figure 1.10. The [Bi6(OH)16]2+ polycations
Ni
-PILCs were also reported by Yamanaka and Brindley [108]. The Ni(NO3)2 solutions were hydrolyzed with NaOH. The NiO pillars were observed after heating the intercalated clay at 500ºC, with a pillar height of ± 5 Å.Cr
Some authors [109-111] reported the intercalation of [Crn(OH)m](3n-m)+ species between smectite layers. The chromia pillars are formed after calcination at 500ºC and exhibit heights of almost 12 Å. The final Cr-PILC seems to have a BET surface area between 350 and 430 m2/g, depending on the preparation conditions.Mg
Attempts were made to introduce polyoxycations of Mg2+ between the clay sheets [112]. [Mg(OH)]+ ions were intercalated and this resulted in basal spacings between 14.6 and 14.9 Å. At 300ºC however, the basal distance dropped to 9.55 Å, which is the thickness of a montmorillonite layer, indicating that these Mg-PILC collapsed and had therefore a poor thermal stability.V
Vanadia pillared montmorillonite catalysts (V-PILC) were synthesized by Choudary et al. [113], by refluxing VOCl3 in benzene with H+-montmorillonite. Rather high interlayer spacings of 13 Å were observed.1.2.6. Mixed Pillaring Species
The use of mixed metal cationic species pillar has certain advantages. The incorporation of specific metal ions into the pillars has an influence on the chemical nature of the final PILC. As a result, catalytic and adsorption properties of the microporous materials can be change in a controlled way. PILC stability is dependent on the stability of the pillaring agent. The structure of the pillar can be changed by using mixed metals and therefore also the pillar height, width, charges and stability varies.
A description of Al/Fe-PILCs is found in different literature [114-116]. These mixed Al/Fe PILCs are particularly active and selective to light olefins during syngas conversion reactions. Other types of mixed alumina pillars are for examples: Al/Cr- PILC [117], Al/Zr-PILC [118], Al/Si-PILC [119], Al/Ga-PILC [120], Al/Mg-PILC [121], Al/La-PILC [122], Al/Ce-PILC [123], (Ce,La)Al-PILC [124], V/Al-PILC and Al/Ru-PILC [125], Fe/Al-PILC [126], AlCeMg-PILC [127], (Sr,Cs)Al/AlFe-PILC [128].
Al13 oligomer, due to its relatively low thermal stability, is not an ideal pillar since industrially used PILC catalysts need frequent regeneration. As was explained earlier, Al13 oligomer has a central Al atom surrounded by twelve edge-sharing (AlO6) octahedra. Thermal stability might be increased by substituting the central Al atom with a larger metal atom to reduce the distortions of the surrounding octahedra. Usually there is no distinction between which type of Al substitution (tetrahedral or octahedral) occurs. Kloprogge and co-workers [129] have an excellent study about tetrahedral

![Figure 1.2. Diagrammatic sketch of the structure of the montmorillonite after Grim [28]](https://thumb-eu.123doks.com/thumbv2/9dokorg/874206.47034/14.918.185.697.390.949/figure-diagrammatic-sketch-structure-montmorillonite-grim.webp)
![Figure 1.3. The condensation of a tetrahedral and octahedral layer results in the formation of so-called TO-clay sheet after Nemecz [27]](https://thumb-eu.123doks.com/thumbv2/9dokorg/874206.47034/15.918.216.778.420.694/figure-condensation-tetrahedral-octahedral-results-formation-called-nemecz.webp)