ENDOTOXIN CONTAMINATION AND in vitro MONOCYTE-MACROPHAGE FUNCTION:
METHODS OF DETECTING, DETOXIFYING, AND ELIMINATING ENDOTOXIN
J. Brice Weinberg
I. INTRODUCTION
Bacterial endotoxin (ET) is a protein - lipopolysaccharide complex that comes from the outer membrane of gram-negative bacteria. Endotoxin has effects on various systems that in- directly or directly affect mononuclear phagocyte function.
Endotoxin can activate the classical and alternate pathways of complement in vivo and in vitro (1, 2 ) . Endotoxin also has direct effects on different cell types including fibroblasts
(3), kidney cells (4), endothelial cells (5), ovary cells (6), B lymphocytes (7), and monocyte-macrophages (8 - 1 1 )0 In ad- dition, through the induction of interleukin 1 [iLl, which is probably the same as lymphocyte-activating factor and endo- genous pyrogen (12)] released from monocyte-macrophage, ET causes changes in T lymphocytes (13), brain cells (14), and synovial cells (15). Although investigators in the past have used ET in the microgram per milliliter range for in vitro studies, recent experience has shown that ET in quantities of
METHODS FOR STUDYING Copyright © 1981 by Academic Press, Inc.
MONONUCLEAR PHAGOCYTES 1 3 9 All rights of reproduction in any form reserved.
ISBN 0-12-044220-5
picogram to nanogram per milliliter has potent effects on m a - crophage function including elaboration of ILl (12) and tissue factor (16) , induction of macrophage-mediated tumor cytotoxici- ty (8 - 1 1 ) , and modulation of macrophage neutral proteinase secretion (17).
The realization that such low quantities of ET can so dramatically influence macrophage function has made investiga- tors more wary of ET contamination in media and various
reagents (10, 1 1 , 18 - 23) . Although the pharmaceutical indus- try is aware of ET ("pyrogen") contamination and produces E T - free products for clinical use, chemical companies do not routinely monitor for the ET content in their products· I and others have previously demonstrated that ET contamination of tissue culture media, sera, and various reagents has dramatic effects on in vitro assays of macrophage-mediated tumor cell killing (9, 1 0 ) . The purpose of this chapter is to describe means of detecting, detoxifying, and eliminating E T .
II. REAGENTS
(1) Glass tubes, 10 x 75 mm borosilicate (Scientific Products, T1290-2)
(2) Sterile plastic pipettes (Falcon Plastics) (3) Serum, adult bovine (Sterile Systems, Inc.) (4) Tissue culture medium: Dulbecco's modified Eagle medium (DMEM) (GIBCO N o . 430-1600) is made from powder using ET-free water. The medium is sterilized by filtration through 0.22 pm diameter pore size Millipore filters. The final medium also contains 20 m M HEPES buffer (Sigma Chemical C o . ) , 1.75 mgm/ml added dextrose (final concentration of dextrose 15 m M ) , 100 U/ml penicillin G potassium (Lilly and C o . ) , and 100 yg/ml streptomycin sulfate (Pfizer)
(5) Water distilling apparatus: Water still AG-2 (Corning Glass Works)
(6) Limulus amebocyte lysate, PanMed, Inc., Three O a k s , Michigan
(7) Chromatography supplies: Sephadex G-200 gel and glass columns (Pharmacia); sodium azide and Tris buffer (Sigma Chemical Co.)
(8) Polymyxin B sulfate, Burroughs Wellcome and Co.
(9) ET-free saline, GIBCO/Invenex Division of Dexter Cor- poration
(10) Drying oven, Thelco Model 1 7 , GCA/Precision Scien- tific
(11) Endotoxin, phenol-extracted Em Coli 0128:B12 (Sigma Chemical Co.)
(12) Human hemoglobin, type IV (Sigma Chemical Co.)
III. PROCEDURES
A. Detection of ET [Limulus amebocyte lysate (LAL) assay]
(1) The lyophilized LAL is dissolved into the prescribed amount of sterile, ET-free water or saline. Unused LAL is aliquotted into 0.2-ml portions and frozen at -20°C for later use. Sterile technique is used.
(2) LAL (0.03 ml) is placed into baked (see Section III.
B. 1) 10 x 75 mm glass tubes, and then 0.03 ml of the sub- stance in question is added to the tubes using separate pi- pettes for all additions. The pH of the sample should be be- tween 6.8 - 7.6 (24), and is adjusted by addition of HC1 or NaOH. The tubes are capped with aluminum foil (which has been previously baked 2 hr at 170°C) to avoid evaporation.
The tubes are kept upright in a rack.
(3) After 60 min of undisturbed incubation in a 37°C water bath, each tube is observed individually for the forma- tion of a clot by smoothly inverting the tube a full 180°.
(4) A positive test is one in which a solid clot is present and does not dislodge on inversion. Increased vis- cosity is considered negative.
(5) Controls should include a negative (commercially bought ET-free water or saline), a positive (commercially bought ET, usually 0.1, 1, 10, and 100 ng/ml), and tests for nonspecific inhibition (mixture of reagent in question with ET). When making serial dilutions of ET, it is important to use fresh pipettes between tubes to avoid carry-over inac- curacies. Table I shows an example of a typical LAL assay with appropriate controls.
(6) Semiquantitation of ET content can be done by serially diluting the positive sample and comparing to a standard curve prepared with ET.
(7) Serum and plasma contain lipoprotein inhibitors of ET, and unless special preparation of the sample is made, the ET content cannot be accurately assessed (24). Although chloro- form extraction will remove the inhibitors, heat treatment is easier and yields comparable results (25). The serum is di- luted 1 : 10 into ET-free saline, heated in a boiling water bath 7.5 min, cooled, centrifuged, and the supernatant is tested as described above (steps 1-6).
Of the various practical methods available to detect the presence of ET [e.g., pyrogenicity in rabbits, death in chicken embryos, and death in sensitized mice (26)], the LAL assay has proved the most useful. The technique described here requires no special equipment and detects >0.5 - 1.0 ng/ml of ET. Other LAL assays using nephelometry (27), determination of precipi-
TABLE I. Typical LAL Assay
Reagenta Result
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
ET-free saline ET, 0.1 ng/ml ET, 0.5 ng/ml ET, 1.0 ng/ml ET, 10.0 ng/ml ET, 100.0 ng/ml Hb, 0.1 \ig/ml Hb, 1.0 \ig/ml Hb, 5.0 \ig/ml Hb, 10.0 ]ig/ml Hb, 50.0 ]ig/ml Hb, 100.0 \ig/ml NaI04, 5 mM
Nal04, 10 mM NaIC>4, 20 mM
NaI04 (5 mM) + ET (1 ng/ml) NaI04 (10 mM) + ET (1 ng/ml) NaI04 (20 mM) + ET (1 ng/ml)
- - + + + + - +
•f
■f
+ + - - -
•f
+ +
aET (endotoxin) , Hb (hemoglobin) , and NaI04 (sodium perio- date) are dissolved into ET-free saline. Numbers 2-6 indi- cate that the LAL is sensitive to >0.5 ng/ml ET. Numbers 7-12 indicate that >g ]}g/ml hemoglobin has the same clotting activity as >0.5 ng/ml ET. Numbers 13-15 imply that 5-20 mM NaI04 has < 0.5 ng/ml ET, and numbers 16-18 indicates that
the negative result of NaI04 is not due to nonspecific inhibi- tion by NaI04 of ET or the LAL reaction.
t a t e d LAL p r o t e i n (28), or determination of amidase a c t i v i t y of LAL (29) may provide more s e n s i t i v e q u a n t i t a t i o n of ET.
D i f f i c u l t y with f a l s e p o s i t i v e s (30) and f a l s e negatives has been minimal in my hands. More p r e c i s e and a c c u r a t e q u a n t i t a - t i o n of ET may be a v a i l a b l e by chemically determining t h e presence of f a t t y a c i d s unique t o ET's ( 3 1 ), but t h e s e n s i t i v i - t y i s not as g r e a t as t h e LAL b i o a s s a y .
B. Detoxification of ET 1. Destruction by Heat
Endotoxin i s r e s i s t a n t t o b o i l i n g and autoclaving (121°C), but dry heat a t 170°C for 2 - 4 h r d e s t r o y s t h e a c t i v i t y of ET (26). Obviously t h i s i s useful only for n o n v o l a t i l e , non-
combustible materials. All of the glassware used is prepared for tissue culture by washing with detergent, drying, and then baking at 170°C for 4 hr for sterilization and ET inactivation purposes.
2. Detoxification by Alkalai Treatment
Alkalai treatment cleaves the fatty acids from the lipid portion of ET and abolishes lipid A activity (32). In some instances when equipment will not tolerate 170°C (e.g., plastic ware), it can be treated with 1 N NaOH at 56°C for 90 min and then washed with ET-free water.
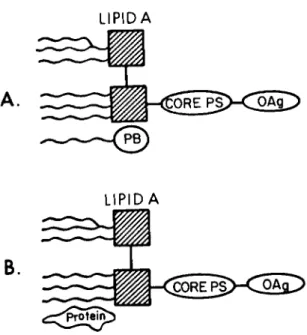
3. Detoxification by Polymyxin B
The amphipathic polypeptide antibiotic polymyxin B binds to lipid A and prevents its actions (33, 34) (Fig. 1). Inclu- sion of 1 - 10 pg/ml polymyxin B in in vitro experiments can counteract the lipid A effects of ET (10, 34 - 36).
4. Use of Lipid A-Resistant Mouse Strains
Some mouse strains including the C3H/HeJ strain are re- sistant to the effects of lipid A (37, 38). By using cells from C3H/HeJ mice, one can avoid lipid A effects. However, other components of ET such as the lipid A protein (LAP) af- fect CßH/HeJ cells as it does cells of lipid A responder mice (39, 40).
Heat at 170°C for 2 - 4 hr and alkali treatment are useful in destroying ET on glassware and other hardware, but they generally are too extreme for use with biological reagents.
Although superficially it would appear that by using polymyxin B-and lipid A-unresponsive mice, one could avoid the problems of ET contamination, this is not so (41). ET with LAP does not bind polymyxin B, and ET with LAP effects lymphocytes and macrophages from C3H/HeJ mice (34, 39-41) (Fig. 1). Since the ET-contaminating reagents may be comparable to ET, which is extracted from bacteria by mild means (38, 41, 42), it would likely contain LAP. Therefore, naturally contaminating ET containing LAP would not be inhibited by polymyxin B, and cells of C3H/HeJ would respond to the ET.
C. Endotoxin-Free Tissue Culture Conditions I. ET-Free Culture Ware
All of the glassware (flasks, beakers, pipettes, etc.) is baked 4 hr at 170°C in metal cannisters (pipettes) or with
LIPIDA
Fig. 1. Diagrammatic representation of endotoxin and lipid A associated protein (B) and endotoxin associating with polymyxin B (A) (45a, 33). The native endotoxin essentially exists as three parts: the polysaccharide (0 antigen), the core polysaccharide containing ΚΏΟ, and the lipid portion
(lipid A), which consists of a diglucosamine backbone with linked fatty acids with or without associated protein. The amount of the hydrophobic lipid A associated protein present in extracted endotoxins depends on the methods used to extract them from the bacteria (38) . The endotoxins frequently exist as large polymers. Polymyxin B (PB) is an amphipathic anti- biotic, which will bind to lipid A and prevent its biological effects (33, 34) . Its binding, however, is apparently inverse- ly proportional to the content of lipid A associated protein, so that endotoxins with a high content of lipid A associated protein are not affected by polymyxin B (40).
aluminum foil covering tops. To date, all sterile plastic ware (Falcon and Costar) I have tested for ET has been nega- tive.
ET-free Water
All liquid tissue culture media that I have bought and tested has contained >1 ng/ml ET (10). Thusr powdered media are formulated with ET-free water and sterilized by filtration.
Many water stills (including those produced by Corning, Bellco,
and Barnstead) and a filtering-reverse osmosis device pro- vided by Millipore will provide ET-free water. Special care must be taken in maintenance of sterile collection tubings and vessels. Low-volume usage encourages contamination.
Some commercial suppliers sell ET-free liquid tissue culture media (e.g., Microbiological Associates).
3. ET-Free Sera
Serum from most commercial suppliers contains ET (10), ap- parently because of postbleeding contamination. Some serum companies will supply ET-free serum (e.g., Sterile Systems, Inc.). Although serum from portal blood of normal people may contain ET (43), serum from nonportal blood of normal animals is ET-free. Serum from patients with liver disease or sepsis may contain ET (43, 44).
D. Elimination of Contaminating ET 1. Removal by Column Chromatography
Endotoxin (as well as smaller biologically active subunits such as lipid A) exists in aqueous solutions as high molecular weight polymers (>1 x 106 dalton) (20, 26, 45, 45a) (see Fig. 1). Thus, sieving chromatography has been used to remove ET from smaller molecular weight substances (10, 20). The dif- ficulty here is to maintain sterile columns to avoid endogenous ET contamination during the procedure.
The column (2.5 x 60 cm glass) is cleaned with detergent, rinsed extensively with ET-free water, dried, assembled, and gas-sterilized with ethylene oxide. Sephadex G-200 gel is boiled, degassed, and poured into the column using sterile technique with the aid of sterile gloves. The column is equi- librated in 0.15 M NaCl, 0.02% sodium azide (as an antibac- terial agent), and 5 mM Tris. All procedures are done at 4°C.
The void volume (containing the ET) is discarded, and fractions containing the protein of interest are dialyzed against ET-free water, and lyophilized. Figure 2 illustrates the separation of ET from soybean trypsin inhibitor using Sephadex G-200 chroma- tography.
2. Removal by Ultracentrifugation
Because of the large polymeric nature of ET, it would be expected to sediment at high g forces leaving smaller proteins in suspension. Although others have been able to remove ET from protein solution using this technique (18, 46), I have not been able to.
70 H
50H
(0 O
-o co
82
3 0 i< δ5
ιοΗ
Blue Dextran Albumin
-/>
Eluant (ml) 120 150
I
180 210 2 4 0 3 0 0 LAL Assay
Degree of
Tumor Killing 4 +
Trypsin Inhibitory Activity (% Original STI Activity)
997%
Fig. 2. Separation of ET from ET-contaminated soybean trypsin inhibitor (STI) by G-200 gel chromatography in 5 mM Tris, 0.15 M NaCl, and 0.02% sodium azide buffer and relation- ship of separated LPS and STI to macrophage tumor cell killing.
Twenty-five milligrams of commercially obtained STI were passed through the 2.5 x 60-cm column at 0.6 ml/min flow rate. Frac- tions of interest were lyophilized before testing. Nonfrac- tionated STI was positive for ET in the LAL assay at >5 ]ig/ml, whereas fractionated STI (postalbumin fraction) was negative through 1000 \xg/ml. The trypsin inhibitory activity of this fraction of STI (tested at 10, 100, and 1000 \ig/ml) was un- altered as compared to the nonfractionated STI, whereas no in- hibitory activity could be found in the void volume fraction
(blue dextran marker). The LAL-negative fraction which con- tained the trypsin inhibitory activity had no influence on macrophage tumor cell-killing activity (neither enhancing nor inhibitory) when tested at 1 to 1000 g/ml, but the LAL posi- tive void fraction, which contained no trypsin inhibitory ac- tivity, made nontumoricidal activated macrophages kill tumor cells in doses as low as 0.5 to 1.0 ng/ml. (From ref. 10, with permission.)
3. Removal by Ultra filtrat ion
Variable success has resulted in attempts to remove ET
from solutions by ultrafiltration (42, 49 - 5 2 ) . Theoretically, the large ET aggregates should be retained behind filters with pore sizes of less than 100,000 dalton cutoff, but this is de- pendent on the solutions used (52). Preliminary studies in my laboratory using Amicon XM100 ultrafiltration membranes indi- cate the possibility of removing ET effectively by multiple passes through these membranes (Weinberg, Naramore, and Capel, manuscript in preparation).
Although gel chromatography separation of ET from reagents is possible, it is very difficult to maintain ET-free condi- tions in columns. Important steps are the inclusion of azide as an antibacterial agent, working at 4°C and essentially con- stant flow of the column buffer when not using the column.
Other methods of ET removal have been tried with limited suc- cess. In using Limulus amebocyte lysate absorption (22, 47), one must cope with incomplete ET removal and inclusion of the LAL into the sample. Polymyxin B affinity columns (48) would not be expected to bind ET containing LAP (see Section III. B.
3 ) . Since erythrocyte membranes bind ET (52a), one might be able to remove ET with erythrocyte absorptions. However, erythrocytes also bind other biological products [including migration inhibitory factor (52a) to type 0 cells] making ac- curate interpretations of some experiments difficult. Ultra- filtration may eventually be the most efficient means of re- moving ET.
IV. CONCLUDING REMARKS
Many reagents used in tissue culture studies of monocytes- macrophages are contaminated with enough ET to effect dramati- cally monocyte-macrophage function. Table II displays results of LAL testing of various agents. In general, protein reagents usually contain ET, and amino acids and salts are usually ET- free. Proteins purified by routine chromatography have always contained large amounts of ET; the "purer" the protein (the more steps in the purification scheme), the more ET that pro- tein is likely to have.
Figure 2 demonstrates how ET contamination of a reagent can alter interpretation of studies of macrophage-mediated tumor cytotoxicity. Contaminating ET could not only give the false impression that a particular contaminated agent has an enhanc- ing effect on macrophage function (e.g., macrophage-mediated tumor cell killing), but the ET could also mask the suppressive
TABLE II. Results of LAL Assay on Various Reagentsa
Positive Negative 1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
Horseradish peroxidase (types II and VI)
Catalase (Sigma) Xanthine oxidase
(grade TV)
Fetuin (types I and II) Bovine pancreatic in- hibitor (type III) Ovomucoid (type II-O)
Soybean trypsin inhibitor (type I-S)
Neuraminidase (type VIII) Hemoglobin (type IV) Porphobilinogen
Superoxide dismutase α-l-Antitrypsin
Bovine serum albumin Concanavalin A Fetal bovine serum
(Gib co)
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
Fetal bovine serum (Flow) L-Histine-HCl
Dime done
Reduced glutathione (grade IV)
Sodium periodate Imidazole (grade III) Dipalmitoylphosphatidyl- choline
Catalase (Boehringer) Phorbol myristate acetate Dextran sulfate
Tissue culture media power (Gibco, Flow) Fetal bovine serum
(Sterile System) Adult bovine serum
(Sterile System)
aReagents 1 - 10 and 17 - 22 were from Sigma; 11 from Truett Laboratories ; 12 and 13 from Miles Laboratories; 14 from Calbiochem; 24 from P-L Biochemicals and Dr. Peter Borchert of Minneapolis, Minnesota; 25 from Pharmacia.
17-21 were tested at 5 mM; 26 was tested after formulation with ET-free water; sera were tested after chloroform extrac- tion; all other agents were tested at 1 mg/ml. (Adopted from reference 10, with permission.)
effects of certain agents. In some experiments the effect of the covert ET could be manifested as an inhibition of a certain function (17). In experiments studying macrophage activation for tumor cytotoxicity, ET and lymphocyte-derived macrophage activating factor act synergistically to enhance macrophage tumoricidal activity (10, 53, 54). Amounts of ET that might be inconsequential in some in vitro assays could produce dra- matic enhancing effects in the presence of macrophage activat- ing factor. Thus, this synergistic activity makes it especial- ly important to use ET-free preparations of lymphokines in studies of macrophage function (54).
Useful tests to determine if the effects of a particular substance are due to ET include heat sensitivity and inhibita-
TABLE III. Effect of Heat and Polymyxin B (PB) on MAF- and/or ET-Induced Tumor Cell Killing by BCG Macrophages from C3H/HeN Micea
n ^ ^ ^ ,,.^. η Degree of tumor cell killing**
25 \ig/ml PBd 0
+++
0 0
++++ 0 0
From reference 10, with permission.
bTumor cell killing quantitated by visual cell counting.
++++ tumor cell killing signifies 0 to 3 tumor cells/300*
microscopic field; +++, 4 to 14 cells/field; ++, 15 to 25 cells/
field; +, 26 to 36 cells/field; and 0, a multilayer of tumor cells over the macrophage. For comparison, the initial tumor cell density (time 0) was 33 to 37 tumor cells/300* field.
cMacrophages treated at 37°C for 2 hr with additives and then additives removed and macrophages challenged with tumor cells. MAF 5%, ET 100 ng/ml. Heating at 80°C for 10 min.
^Polymyxin B present in 2-hr pretreatment only.
bility by polymyxin B (10, 41). Table III shows how heat and polymyxin B help distinguish between the effects of ET and macrophage-activating factor in assays of macrophage-mediated tumor cell killing. If heating an agent to temperature known to denature or inactivate it does not change its effect on macrophage function, the effect is probably due to contaminating ET. If the effect of an agent can be blocked by polymyxin B, that effect is most likely due to ET. However, the effects of ET-contaminated agents that are not inhibitable by polymyxin B could still be due to the contaminating ET with high LAP con- tent, since ET with LAP is not inhibited by polymyxin B. For practical purposes, meaningful evaluations of the effects of agents on macrophage function can only be obtained when those agents are free of ET as determined by the LAL assay.
Although maintenance of ET-free culture conditions dra- matically increases the work and complexity of operations, it is necessary for the accurate interpretation of in vitro ex- periments investigating monocyte-macrophage function.
rj.cujLcauj(«:iJL. Q U U J . U X V C ;
O PB Medium (DMEM)
Macrophage activating (MAF)
Heated MAF ET
Heated ET MAF + ET
Heated MAF + ET
factor 0
+++
0 ++++
++++
++++
++++
1 (3 x 10 ) peritoneal cells per chamber.
REFERENCES
1. D. C. Morrison and L. F. Kline. Activation of the classi- cal and properdin pathways of complement by bacterial lipopolysaccharide (LPS) . J. Immunol. 118: 362-368, 1977.
2. V. E. Gilbert and A. I. Braude. Reduction of serum com- plement in rabbits after injection of endotoxin. J. Exp.
Med. 116: 477-490f 1962.
3. A. Vaheri, E. Ruoslahti, M. Sarvas, and M. Nurminen.
Mitogenic effect by lipopolysaccharide and pokeweed lec- tin on density-inhibited chick embryo fibroblasts. J.
Exp. Med. 138: 1356-1364, 1973.
4. A. McGivney and S. G. Bradley. Effects of bacterial en- dotoxin on lysosomal and mitochondrial enzyme activities of established cell cultures. J. Reticuloendothel. Soc.
26: 307-316, 1979.
5. H. S. Rubenstein, J. Fine, and A. H. Coons. Localization of endotoxin in the walls of the peripheral vascular sys- tem during lethol endotoxenus. Proc. Soc. Exp. Biol. Med.
Ill: 458-467, 1962.
6. K. W. Brunson and G. L. Nicolson. Lipopolysaccharide ef- fects on sensitive and resistant variant Chinese hamster ovary cell lines. J. Supramolec. Structr. 9: 231-242, 1978.
7. J. Andersson, F. Melchers, C. Galanos, and 0. Luderity.
The mitogenic effect of lipopolysaccharide on bone-marrow- derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J. Exp. Med. 137: 943-953, 1973.
8. P. Alexander and R. Evans. Endotoxin and double-stranded RNA render macrophages cytotoxic. Nature (New Biol.) 232:
76-78, 1971.
9. J. B. Hibbs, Jr., R. R. Taintor, H. A. Chapman, Jr., and J. B. Weinberg. Macrophage tumor killing: Influence of the local environment. Science 197: 279-282, 1977.
10. J. B. Weinberg, H. A. Chapman, Jr., and J. B. Hibbs, Jr.
Characterization of the effects of endotoxin on macro- phage tumor cell killing. J. Immunol. 121: 72-80, 1978.
11. S.W. Rüssel, W. F. Doe, and A. T. Mclntosh. Functional characterization of a stable, noncytolytic stage of macro- phage activation in tumors. J. Exp. Med. 146: 1511-1520, 1977.
12. Anon. Revised nomenclature for antigen-nonspecific T cell proliferation and helper factors. J. Immunol. 123:
2928-2929, 1979.
13. I. Gery and B. H. Waksman. Potentiation of lymphocyte re- sponses to mitogens. II. The cellular source of potentiat- ing mediators. J. Exp. Med. 136: 143-155, 1972.
14. W. I. Cranston. Central mechanisms of fever. Fed. Proc.
38: 49-51, 1979.
15. J. M. Dayer, J. H. Passwell, E. E. Schneeberger, and S. M. Krane. Interactions among rheumatoid synovial cells and monocyte-macrophages; production of collagenase-
stimulating factor by human monocytes exposed to conca- navalin A or immunoglobulin Fc fragments. J. Immunol.
124: 1712-1720, 1980.
16. F. R. Rickles, J. Levin, J. A. Hardin, C. F. Barr, and M. E. Conrad, Jr. Tissue factor generation by human mo- nonuclear cells: Effects of endotoxin and dissociation of tissue factor generation from mitogenic response. J.
Lab. Clin. Med. 89: 792-803, 1977.
17. H. A. Chapman, Jr., Z. Vavrin, and J. B. Hibbs, Jr.
Modulation of plasminogen activator secretion by acti- vated macrophages: Influence of serum factors and cor- relation with tumoricidal potential. Proc. Nat. Acad.
Sei. 76: 3899-3903, 1979.
18. H. F. Dvorak and R. C. Bast, Jr. Nature of the iramunogen in crystalline serum albumins. Immunochemistry 7: 118- 124, 1970.
19. K. W. Brunson and D. W. Watson. Concanavalin A prepara- tions with activities related to bacterial lipopoly- saccharide. J. Immunol. 115: 599-600, 1975.
20. M. Loos, S. Vadlamudi, M. Meltzer, S. Shifrin, T. Borsos, and A. Goldin. Detection of endotoxin in commercial L-aspariginase preparation by complement fixation and separation by chromatography. Cancer Res. 32: 2292- 2296, 1972.
21. L. Z. Bito. Inflammatory effects of endotoxin-like con- taminants in commonly used protein preparations. Science 196: 83-85, 1977.
22. S. E. Graber, J. D. Bomboy, Jr., W. D. Salmon, Jr., and S. B. Krantz. Evidence that endotoxin is the cyclic 3*:5'-GMP-promoting factor in erythroprotein preparations.
J. Lab. Clin. Med. 93: 25-31, 1979.
23. D. Fumarola and E. Jirillo. Endotoxin contamination of some commercial preparations used in experimental re- search. In "Biomédical Applications of the Horseshoe Crab (Limulidae)" (E. Cohen, F. B. Bang, J. Levin, J. J.
Marchalonis, T. G. Pistole, R. A. Prendergrast, C.
Shuster, Jr., and S. W. Watson, eds.), pp. 379-385.
Alan R. Liss, New York, 1979.
24. J. Levin, P. A. Tomasulo, and R. S. Oser. Detection of endotoxin in human blood and demonstration of an in- hibitor. J. Lab. Clin. Med. 75: 903-911, 1970.
25. J. J. Corrigan, Jr., and J. F. Kiernat. Effect of poly- myxin B sulfate on endotoxin activity in a gram-negative septicemia model. Pediat. Res. 13: 48-51, 1979.
26. K. C. Milner, J. A. Rudbach, and E . Ribi. Bacterial en- dotoxins. General characteristics. In "Microbial Toxins IV" (G. Weinbaum, S. Kadis, and S. I. Ajl, e d s . ) , pp. 1-65. Academic Press, New York, 1971.
27. V. P. Hollander and W. C. Harding. A sensitive spectro- photometric method for measurement of plasma endotoxin.
Biochem. Med. 15: 28-33, 1976.
28. R. Nandan and D. R. Brown. An improved in vitro pyrogen test: To detect picograms of endotoxin contamination in intravenous fluids using Limulus amebocyte lysate.
J. Lab. Clin. Med. 89: 910-918, 1977.
29. T. Harada, T. Morita, S. Iwanga, S. Nakamura, and M.
Neiva. A new chromogenic substrate method for assay of bacterial endotoxins using Limulus hemocyte lysate. In
"Biomédical Applications of the Horseshoe Crab (Limulidae)"
(E. Cohen, F. B. Bang, J. Levin, J. J. Marchalonis, T. G.
Pistole, R. A. Prendergrast, C. Shuster, Jr., and S. W . Watson, e d s . ) , p p . 209-220. Alan R. Liss, New York, 1979.
30. R. J. Elin and S. M. Wolff. Nonspecificity of the Limulus amebocyte lysate test: Positive reactions with polynucleotides and proteins. J. Infect. Dis. 128:
349-352, 1973.
31. S. K. Maitra, M. C. Schotz, T. T. Yoshikawa, and L. B.
Guze. Determination of lipid A and endotoxin in serum by mass spectroscopy. Proc. Nat. Acad. Sei. 75: 3993- 3997, 1978.
32. D. Tripodi and A. Nowotny. Relation of structure to func- tion in bacterial O-antigens. V. Nature of active sites in endotoxic lipopolysaccharides of Serratla marcescens.
Ann. NY Acad. Sei. 133: 604-621, 1966.
33. D. C. Morrison and D. M. Jacobs. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides.
Immune-chemistry 13: 813-818, 1976.
34. D. M. Jacobs and D. C. Morrison. Inhibition of the mito- genic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J. Immunol. 118: 21-27, 1977.
35. W. F. Doe, S. T. Yang, D. C. Morrison, S. J. Betz, and P. M. Henson. Macrophage stimulation by bacterial lipo- polysaccharides. I I . Evidence for independent differentia- tion signals delivered by lipid A and a protein rich frac- tion of LPS. J. Exp. Med. 148: 557-568, 1978.
36. L. P. Ruco and M. S. Meltzer. Macrophage activation for tumor cytotoxicity: Tumoricidal activity by macrophages from CßH/HeJ mice requires at least two activation stimu- li. Cell. Immunol. 41: 35-51, 1978.
37. B. M. Sultzer. Genetic control of leucocyte responses to endotoxin. Nature 219: 1253-1254, 1968.
38. B. J. Skidmore, D. C. Morrison, J. M . Chiller, and W . 0.
Weigle. Immunologie properties of lipopolysaccharide
(LPS). II. The unresponsiveness of C3H/HeJ mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J. Exp. Med. 142: 1488-1508, 1975.
39. B. M. Sultzer and G. W. Goodman. Endotoxin protein: A B-cell mitogen and polyclonal activator of C3H/HeJ lym- phocytes. J. Exp. Med. 144: 821-827, 1976.
40. D. C. Morrison, S. J. Betz, and D. M. Jacobs. Isolation of a lipid A bound polypeptide responsible for "LPS-
initiated" mitogenesis of C3H/HeJ spleen cells. J. Exp.
Med. 144: 840-846, 1976.
41. D. C. Morrison and B. J. Curry. The use of polymyxin B and C3H/HeJ mouse spleen cells as criteria for endotoxin contamination. J. Immunol. Meth. 27: 83-92, 1979.
42. D. C. Morrison and L. Leive. Isolation of lipopoly- saccharides from bacteria. Methods Enzymol. 28B: 254- 262, 1972.
43. H. Ravin, D. Rowley, C. Jenkins, and J. Fine. On the absorption of bacterial endotoxin from the gastrointes- tinal tract of the normal and shocked animal. J. Exp.
Med. 112: 783-792, 1960.
44. J. Levin. The Limulus test: A status report. In
"Biomédical Applications of the Horseshoe Crab (Limulidae)"
(E. Cohen, F. B. Bang, J. Levin, J. J. Marchalonis, T. T.
Pistole, R. A. Prendergrast, C. Shuster, Jr., and S. W.
Watson, eds.), pp. 235-244. Alan R. Liss, New York, 1979.
45. J. A. Cameron. Bacterial lipopolysaccharide as a void volume marker for agarose gel permeation chromatography.
J. Chromatogr. 37: 331-332, 1968.
45a. C. Galanos, 0. Luderitz, E. T. Rietschel, and 0. Westpal.
Newer aspects of the chemistry and biology of bacterial lipopolysaccharides, with special reference to their lipid A component. Int. Rev. Biochem. 14: 239-335, 1977.
46. R. K. Shadduck, A. Waheed, A. Porcellini, V. Rizzoli, and J. Levin. A method for removal of endotoxin from purified colony stimulating factor. Proc. Soc. Exp. Biol. Med.
164: 40-50, 1980.
47. S. E. Siegel, N. Shore, J. Ortega, and P. P. Dukes. Re- moval of endotoxin (pyrogen) from erythropoietin by Limulus amebocyte lysate. Fed. Proc. 33: 608, 1974.
48. D. C. Morrison, J. F. Roser, C. G. Cochrane, and P. M.
Henson. The initiation of mast cell degranulation: Ac- tivation of the cell membrane. J. Immunol. 114: 966-970, 1975.
49. J. C. Craddock, L. A. Guder, D. L. Francis, and S. L.
Morgan. Reduction of pyrogens-application of molecular filtration. J. Pharm. Pharmacol. 30: 198-199, 1978.
50. J. Hattingh, H. Laburn, and D. Mitchell. Fever induced in rabbits by intravenous injection of bovine serum
albumin. J. Physiol. 290: 69-77, 1979.
51. L. W. Henderson and E. Beans. Successful production of sterile pyrogen-free electrolyte solution by ultrafiltra- tion. Kidney Int. 14: 522-525, 1978.
52. K. J. Sweadner, M. Forte, and L. L. Nelson. Filtration removal of endotoxin (pyrogens) in solution in different states of aggregation. Appl. Environ. Microbiol. 34:
382-385, 1977.
52a. G. F. Springer, J. C. Adye, A. Bezkorovainy, and B. Jir- gensons. Properties and activity of the lipopoly-
saccharide-receptor from human erythrocytes. Biochemistry 13: 1379, 1974.
52b. R. A. Fox, J. M. MacSween, and R. L. McGuire. Potentia- tion of the macrophage response to migration inhibition factor from fetal calf serum by blood group substances with human H activity. Scand. J. Immunol. 5: 941, 1976.