1
2
3
Alterations in the Activity of Spinal and Thalamic Opioid Systems
4
in a Mice Neuropathic Pain Model
5 Ewelina Rojewska,aAgnieszka Wawrzczak-Bargiela,bEdina Szucs,cSandor Benyhe,cJoanna Starnowska,a
6 Joanna Mika,aRyszard Przewlockiband Barbara Przewlockaa*
7 aDepartment of Pain Pharmacology, Institute of Pharmacology, Polish Academy of Sciences, 12 Smeztna Street, 31-343 Krakow, Poland 8 bDepartment of Molecular Neuropharmacology, Institute of Pharmacology, Polish Academy of Sciences, 12 Smeztna Street, 31-343 Krakow, Poland 9 cInstitute of Biochemistry, Biological Research Center, Hungarian Academy of Sciences, Temesvarikrt 62 Street, Szeged 6726, Hungary
10
11 Abstract—Clinical studies have reported lower effectivity of opioid drugs in therapy of neuropathic pain. There- fore, to determine the changes in endogenous opioid systems in this pain more precisely, we have studied the changes in the pain-related behavior on days 1, 14, and 28 following a chronic constriction injury (CCI) to the sci- atic nerve in mice. In parallel, we have studied the changes of -(MOP), -(DOP) and -(KOP) receptors, proenkephalin (PENK) and prodynorphin (PDYN) mRNA levels, as well as GTPcS binding of opioid receptors on the ipsi- and con- tralateral parts of the spinal cord and thalamus on the 14th day following CCI, as on this day the greatest mani- festation of pain-related behavior was observed. On ipsilateral spinal cord, the decrease in MOP/DOP/KOP receptor and increase in PDYN/PENK mRNA expression was observed. In thalamus, MOP/DOP/KOP receptor expression decreased contralaterally. On ipsilateral side, there were no changes in PDYN/PENK or DOP/KOP receptor expression, but MOP mRNA decreased. The spinal GTPcS binding of MOP/DOP/KOP receptor ligands decreased on the ipsilateral side, yet the effect was less pronounced for DOP receptor ligands. In thalamus, a decrease was observed on the contralateral side for all opioid receptor ligands, especially for DOP ligand. A less pronounced decrease in GTPcS binding of spinal DOP ligands may indicate a weaker stimulation of ascending nociceptive pathways, which could explain the absence of decreased activity of DOP receptor ligands in neuropa- thy. These findings may suggest that drugs with a higher affinity for the DOP receptor will perform better in neu- ropathic pain.Ó2018 Published by Elsevier Ltd on behalf of IBRO.
Key words: chronic constriction injury, neuropathic pain, opioid peptides, opioid receptors.
12 INTRODUCTION
13 Neuropathic pain tends to be less opioid responsive than
14 nociceptive pain, and opioids are only partially effective in
15 preclinical models of neuropathic pain, and this
16 phenomenon is not fully understood. The analgesic
17 properties of opioid drugs in neuropathic pain may
18 depend on molecular changes in endogenous opioid
19 systems that contribute to the development and
20 maintenance of this type of pain by weaker
21 counteracting pain stimulation. Any nerve tissue injury
22 leads to endogenous changes on the molecular and
23 systemic levels, with antinociceptive systems being
24 rapidly activated just after injury, and losing their initial
25 analgesic efficacy shortly after. The widespread
changes, including intensified release of pronociceptive 26
molecules, counteract the action of exogenous opioids; 27
this, with weakened efficacy of endogenous 28
antinociceptive systems themselves, contributes to 29
unsatisfying analgesic effect of opioid drugs, which are 30
eventually far less efficient in neuropathic pain than in 31
nociceptive pain. It has been shown in a number of 32
studies that the activity of endogenous opioid peptides 33
is changed by nociceptive and chronic painful stimuli; 34
the increase in endogenous opioid peptide release 35
consequently enhances opioid receptor occupancy. This 36
effect has been documented in animal and human 37
studies (Albe-Fessard et al., 1985; Iadarola et al., 1988; 38
Zangen et al., 1998; Zubieta et al., 2001; Bencherif 39
et al., 2002; Obara et al., 2009; Mika et al., 2014; 40
Popiolek-Barczyk et al., 2014). Neuropathic pain is asso- 41
ciated with significant changes in spinal and thalamic neu- 42
ronal activity and sensitization of the neural structures 43
involved in pain perception (Patel and Dickenson, 2016; 44
Sandkuler et al., 2011), which may play a key role in the 45
persistent experience of neuropathic pain in humans. 46
https://doi.org/10.1016/j.neuroscience.2018.08.013
0306-4522/Ó2018 Published by Elsevier Ltd on behalf of IBRO.
*Corresponding author. Address: Institute of Pharmacology, Polish Academy of Sciences, Department of Pain Pharmacology, 12 Smetna Str., 31-343 Cracow, Poland. Fax: +48-12-6374500.
E-mail address:przebar@if-pan.krakow.pl(B. Przewlocka).
Abbreviations:CCI, chronic constriction injury; EGTA, ethylene glycol- bis(b-aminoethyl ether)-N,N,N0,N0-tetraacetic acid; PDYN, prodynorphin; PENK, proenkephalin.
RESEARCH ARTICLE
E. Rojewska et al. / Neuroscience xxx (2018) xxx–xxx
1
47 Changes characteristic of neuropathic pain may also be
48 induced in animal models (Henderson et al., 2013). Opi-
49 oid peptides and their receptors are normally involved in
50 mechanisms blocking out pain, and their functional and
51 biochemical alterations appear to display a critical role
52 in the development and maintenance of neuropathic pain.
53 Our previous studies have shown a decrease in the
54 expression of opioid receptors in the ipsilateral part of
55 the spinal cord and DRG in rat and mouse models of
56 neuropathy (Obara et al., 2009; Mika et al., 2014;
57 Popiolek-Barczyk et al., 2014). Interestingly, in patients
58 suffering from chronic central pain, a positron-emission
59 tomography study has shown a decrease in opioid [11C]
60 diprenorphine binding in the thalamus contralaterally to
61 the painful side (Maarrawi et al., 2007b). This study was
62 however, limited only to the MOP receptor; but because
63 opioids have different affinities for opioid receptors, it is
64 important to know which receptor profile of the opioid drug
65 is the most suitable for the treatment of neuropathy. And
66 therefore, studying changes in individual types of opioid
67 receptors may help indicate such a drug.
68 An injury of a nervous tissue leads to complex
69 changes in ascending tracts, from peripheral nervous
70 system, through spinal cord, to brain structures. Our aim
71 was to verify whether inflicting an injury just on one site
72 of the nervous system would cause uni- or bilateral
73 changes, and whether the changes are similar in the
74 spinal cord and in the brain tracts. That is why we
75 focused on the issue, as it could potentially explain
76 some aspects of neuropathic pain development and
77 opioid drugs action. Recently, asymmetry in different
78 functions in the brain in neuropathic pain has been
79 suggested (Leite-Almeida et al., 2014), and this problem
80 needs to be addressed more extensively in animal neuro-
81 pathic pain models.Obara et al. (2010)demonstrated that
82 differences in the pharmacological effectiveness of differ-
83 ent opioid receptor ligands with peptide and nonpeptide
84 chemical structures in neuropathic pain could result from
85 functional changes in the -(MOP) receptor in the spinal
86 cord and DRG in a chronic constriction injury (CCI) rat
87 model and Narita et al. (2002) have demonstrated that
88 nerve injury leads to a decrease in MOP receptor-
89 mediated G-protein activation in the spinal cord and in
90 the brain. Little is known, however, about the functional
91 alterations of KOP and DOP receptors in the nociceptive
92 pathways upon neuropathic pain. This is of particular
93 interest, since opioids with particular (differential) selectiv-
94 ity for opioid receptor types may be more suited for treat-
95 ment of neuropathic pain. In our current research we have
96 used besides MOP-, -(DOP) and -(KOP) mRNA level, the
97 selective opioid receptor ligand-stimulated guanosine-50-
98 o-(3-thio) triphosphate (GTPcS) binding to measure the
99 activation of G-proteins. This method characterizes the
100 functional state of the receptor and provides convenient
101 measures of opioid receptor activity close to the receptor
102 in the signaling cascade. The results presented in this
103 paper extend earlier observations (Zhang et al., 1998;
104 Xiao et al., 2008; Obara et al., 2009) and provide addi-
105 tional impact on our understanding of the mechanisms
106 of reduced antinociceptive effectiveness of opioids under
107 neuropathic pain conditions.
Therefore, in the present paper, we analyzed 108
neuropathic pain-related behavioral changes estimated 109
1, 14, and 28 days after sciatic nerve injury. In parallel, 110
we have studied the changes of MOP, DOP and KOP 111
receptors, proenkephalin (PENK) and prodynorphin 112
(PDYN) mRNA levels, as well as GTPcS binding of 113
opioid receptors on the ipsi- and contralateral parts of 114
the spinal cord and thalamus on the 14th day following 115
CCI, as on this day the most pronounced behavioral 116
changes were observed. 117
EXPERIMENTAL PROCEDURES 118
Animals 119
Adult male Albino-Swiss CD-1 mice (Charles River, 120
Germany; 20–25 g) were used in this study. Animals 121
were housed in groups of six in cages with sawdust 122
bedding under a standard 12 h/12 h light/dark cycle 123
(lights on at 06.00 a.m.); food and water were available 124
ad libitum. All experiments were carried out according to 125
the recommendations of International Association for the 126
Study of Pain (Zimmermann, 1983) and the NIH Guide 127
for Care and Use of Laboratory Animals and were 128
approved by the Local Bioethics Committee (Krakow, 129
Poland, permission numbers 1214/2015). 130
Chronic constriction injury (CCI) 131
The CCI model was performed according toBennett and 132
Xie (1988). The surgical procedure was performed under 133
isoflurane anesthesia. Briefly, an incision was made 134
below the right hipbone, parallel to the sciatic nerve. 135
The sciatic nerve was exposed, and three ligatures (4/0 136
silk) were tied loosely around the nerve distal to the sciatic 137
notch with 1-mm spacing, until a brief twitch in the respec- 138
tive hind limb was observed. After CCI, all mice developed 139
tactile/thermal hypersensitivity. The mice with sciatic 140
nerve injury will be referred to by the abbreviation ‘‘CCI 141
mice” throughout the text of the manuscript. The beha- 142
vioral experiments were conducted on the 1st, 14th and 143
28th day following the CCI surgical procedure. Biochemi- 144
cal experiments were conducted on the 14th day after 145
injury, the day of major changes in response to thermal 146
and mechanical stimuli. 147
Behavioral tests 148
Von Frey’s test. Mechanical tactile hypersensitivity in 149
CCI mice was measured on the 1st, 14th and 28th day 150
after CCI using a series of von Frey filaments (Stoelting, 151
Wood Dale, IL, USA), ranging from 0.6 to 6 g (Mika 152
et al., 2015). Animals were placed in plastic cages with 153
a wire-mesh floor, allowing them to move freely. They 154
were allowed to acclimate to this environment for approx- 155
imately 5–15 min prior to testing. The von Frey filaments 156
were applied in ascending order to the midplantar surface 157
of the both hind paw through the mesh floor. Each probe 158
was applied to the foot until it started to bend. The ipsilat- 159
eral and contralateral paws in CCI mice (or both hind 160
paws in naı¨ve mice) were tested 2–3 times and a mean 161
162 value was calculated. The time interval between consec-
163 utive applications of filaments was at least 5 s.
164 Cold plate test. Sensitivity to noxious thermal stimuli
165 was assessed on the 1st, 14th and 28th day after CCI
166 using a Cold/Hot Plate Analgesia Meter from Columbus
167 Instruments. The latency was defined as the amount of
168 time it took for the hind paw to begin to shake after the
169 mouse was placed on a cold plate (2°C). In CCI mice,
170 the injured paw reacted first in all cases. The ipsilateral
171 paw reaction was noted first and then the contralateral
172 paw response was awaited and noted. In naive mice the
173 reaction of any hind paw was noted. The cut-off latency
174 for this test was 30 s (Mika et al., 2015).
175 Biochemical study
176 qRT-PCR analysis. RNA extraction and comple-
177 mentary DNA (cDNA) synthesis. On day 14 after CCI,
178 when the most pronounced changes in response to
179 thermal and mechanical stimuli were observed, the mice
180 were decapitated. Immediately after decapitation the
181 spinal cord was removed using hydraulic pressure and
182 the brain was dissected from the skull. Tissue was
183 collected on ice-cold plate. Spinal cord lumbar
184 fragments (L4–L6) were divided for ipsi- and
185 contralateral parts. The thalamus was dissected
186 according to Palkovits and Brownstein (1987). At first
187 the hypothalamus, cerebellum, cortex hippocampus and
188 mesencephalon tissues were dissected. Finally the thala-
189 mus was dissected from the rest of remaining tissue and
190 divided on ipsi- and contralateral parts to the site of sciatic
191 nerve injury.
192 Total RNA was extracted according to the method
193 described byChomczynski and Sacchi (1987)using TRI-
194 zol reagent (Invitrogen) as previously described
195 (Rojewska et al., 2016). The tissue samples were placed
196 in individual tubes containing the tissue storage reagent
197 RNA later (Ambion Inc.) and were stored at 70°C for
198 RNA isolation. For cDNA synthesis, 1000 ng of total
199 RNA was reverse transcribed using an Omniscript RT
200 Kit (Qiagen) with oligo(dT) primer (Fermentas) in a total
201 reaction volume of 20ll. The cDNA was diluted 1:10 with
202 H2O, and for each reaction, approximately 50 ng of cDNA
203 synthesized from the total RNA template was obtained
204 from each individual animal and used for quantitative
205 real-time polymerase chain reaction (qRT-PCR). qRT-
206 PCR was performed using Assay-On-Demand TaqMan
207 probes (Applied Biosystems, USA) and run on a Real-
208 Time PCR iCycler (Bio-Rad, Hercules, CA, USA). The
209 amplification efficiency for each assay was determined
210 by running a standard dilution curve. The following Taq-
211 Man primers were used: rat hypoxanthine guanine phos-
212 phoribosyltransferase, (Mm03024075_m1; HPRT1),
213 PDYN (Mm00457573_m1; Pdyn); preproenkephalin
214 (Mm01212875_m1; Penk); opioid receptor, mu 1
215 (Mm01188089_m1; Oprm1, MOP); opioid receptor, delta
216 1 (Mm01180757_m1; Oprd1, DOP); and opioid receptor,
217 kappa 1 (Mm01230885_m1; Oprk1, KOP). The Hprt
218 levels did not significantly differ across all groups, and
219 Hprt was, therefore, used as a housekeeping gene control
(data not shown). The cycle threshold values were calcu- 220
lated automatically with the iCycler IQ 3.0 software using 221
the default parameters. The RNA abundance was calcu- 222
lated as 2(threshold cycle). 223
GTPcS functional binding assay 224
Chemicals. The highly selective MOP receptor agonist 225
enkephalin analog Tyr-D-Ala-Gly-(NMe)Phe-Gly-ol 226
(DAMGO) and the KOP receptor agonist peptide 227
dynorphin1–13 were obtained from Bachem Holding AG 228
(Bubendorf, Switzerland). The structurally modified DOP 229
receptor-specific deltorphin II derivative, Ile5,6deltorphin 230
II (Tyr-D-Ala-Phe-Gly-lle-lle-Gly-NH2) was synthesized 231
in the Laboratory of Chemical Biology of the Biological 232
Research Centre (BRC, Szeged, Hungary). Each ligand 233
was dissolved in tri distilled water and stored in 1 mM 234
stock solution at 20°C. EGTA, MgCl26H2O, NaCl, 235
Tris–HCl, guanosine 50-diphosphate sodium salt (GDP) 236
and guanosine 50-O-[c-thio]triphosphate salt (GTPcS) 237
were purchased from Sigma–Aldrich (Budapest, 238
Hungary). The radiolabeled GTP analog [35S]GTPcS 239
(specific activity: 3.71013 Bq/mmol; 1000 Ci/mol) was 240
obtained from Hartmann Analytic (Braunschweig, 241
Germany). The Ultima GoldTM MV harmless scintillation 242
cocktail was purchased from Perkin Elmer. 243
GTPcS binding. Mouse spinal cord and thalamus for 244
G-protein binding assays were collected only on day 14 245
after CCI, when the most pronounced changes in 246
response to thermal and mechanical stimuli were 247
observed. The structures were prepared as described 248
for the mRNA assay in the section above. The crude 249
membrane fractions of mouse spinal cord and thalamus 250
were used for [35S]GTPcS binding experiments after 251
being prepared as described earlier (Sz}ucs et al, 2016). 252
Briefly, the thawed and ice-cooled spinal cord and thala- 253
mus were homogenized on ice in ten volume (10 ml buf- 254
fer/g original tissue) ice-cold TEM buffer (50 mM Tris– 255
HCl, 1 mM EGTA, 5 mM MgCl2, pH 7.4). Protein concen- 256
trations were determined by the Bradford method 257
(Bradford, 1976) and were approximately 4–6 mg/ml. 258
Membrane samples were then aliquoted into the Eppen- 259
dorf tubes containing between 50 and 60ml of membrane 260
suspensions and stored at80°C until further 261
processing. 262
Opioid ligand-stimulated GTPcS functional binding 263
assay. In GTPcS binding experiments, the GDPc-GTP 264
exchange of the Gai/o proteins was measured in the 265
presence of the ligands to determine their potency and 266
the maximal efficacy of the activated G-proteins. The 267
functional [35S]GTPcS binding experiments were 268
performed as previously described (Traynor and 269
Nahorski, 1995). Briefly, the membrane proteins 270
(10mg/ml) were incubated at 30°C for 60 min with[35S]- 271
GTPcS (20 MBq/0.05 cm3; 0.05 nM) and increasing con- 272
centrations (1010–105M) of DAMGO in Tris–EGTA 273
buffer (pH 7.4) containing 30mM GDP, 1 mM EGTA, 274
5 mM MgCl2, 100 mM NaCl and 50 mM Tris–HCl in a final 275
volume of 1 ml/reaction tube. Nonspecific binding was 276
277 determined with 10mM of unlabeled GTPcS and sub-
278 tracted from the total binding. Basal activity (defined as
279 100%) indicates constitutive G-protein activity levels in
280 the absence of any stimulating ligand. Bound and free
281 [35S]GTPcS were separated by vacuum (Brandel M24R
282 Cell Harvester) filtration through the Whatman GF/B glass
283 fiber filters and washed three times with 5 ml of ice-cold
284 50 mM Tris–HCl (pH 7.4) buffer. The analyses were per-
285 formed in triplicate and repeated at least three times.
286 Increasing concentrations of the ligands produced
287 dose-dependent stimulation of [35S]GTPcS binding in
288 each sample. High activation of G-proteins in MOP
289 (DAMGO) and KOP (dynorphin1–13) receptors were
290 observed in the spinal cord and thalamus of naive
291 animals on both the contralateral and ipsilateral sides.
292 Moderate stimulations by Ile5,6deltorphin II were found in
293 the spinal cord and thalamus for the DOP receptor.
294 Data analysis
295 The behavioral data are presented as mean ± S.E.M. of
296 9–15 mice per group. Inter-group differences were
297 analyzed by ANOVA followed Bonferroni’s multiple
298 comparison test. Significance was defined as
299 ***p< 0.001 indicating a significant difference compared
300 with the control (naive) animals;ooop< 0.001 indicating
301 a significant difference compared with the contralateral
302 side.
303 The qRT-PCR data are presented as the fold change
304 of the controls, which represents normalized averages
305 derived from the threshold cycles in qPCR and from 4 to
306 10 samples per group. Inter-group differences were
307 analyzed by Bonferroni’s multiple comparison test.
308 Significance was defined as *p< 0.05, **p< 0.01
309 indicating a significant difference compared with the
310 control (naive) animals. All graphs were prepared using
311 GraphPad Prism 7.0.
312 Data analysis of GTPcS binding was performed with
313 GraphPad Prism 5.0 software (GraphPad Prism
314 Software Inc., San Diego, CA, USA). Non-linear
315 regression analysis of the ligand-stimulated [35S]GTPcS
316 binding assays used the ‘sigmoidal dose–response’
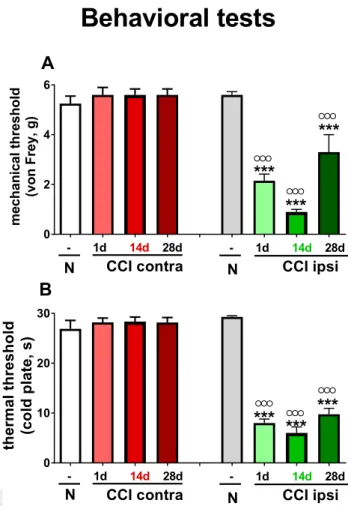
317 fitting to determine the maximal stimulation or efficacy
318 (Emax) of the receptors’ G-protein and ligand potency
319 (EC50). Stimulation is represented as a percent of the
320 specific [35S]GTPcS binding observed above the basal
321 activity level (taken to be 100%). Unpaired t-tests with
322 two-tailed P-values were performed to determine
323 significance using GraphPad Prism 5.0.
324 RESULTS
325 Time-course changes in sensitivity to mechanical
326 and thermal stimuli as measured in naı¨ve mice and 1,
327 14 and 28 days after injury in CCI mice
328 Pain thresholds in response to mechanical and thermal
329 stimuli were measured by the von Frey and cold plate
330 tests, respectively. No changes in the response to both
331 types of stimuli were observed on the contralateral paw
332 at the time points examined (Fig. 1A, B) as compared to
naı¨ve mice. In contrast, response times on the 333
ipsilateral side of the injury were significantly reduced 334
starting from the very first day after CCI. The lowest 335
pain threshold in the von Frey test was observed on day 336
14. On day 28, the threshold reached values closest to 337
the level of controls, although there was still an 338
observable significant decrease in the pain threshold 339
(Fig. 1A). The response time to thermal stimuli was also 340
significantly reduced at all measured time points. 341
Differences between time points were not large, yet the 342
strongest effect was observed on day 14 after nerve 343
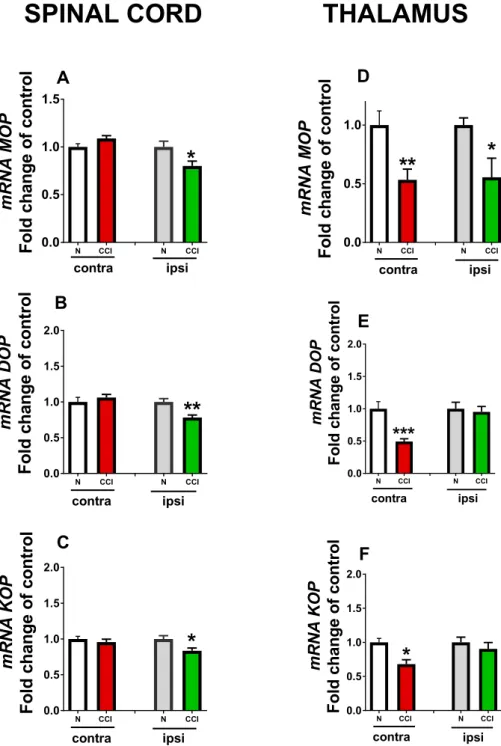
injury (Fig. 1B). 344
The level of opioid receptors’ mRNA in the spinal 345
cord and thalamus measured in naı¨ve mice and 346
14 days after injury in CCI mice 347
The levels of MOP, DOP and KOP opioid receptor mRNA 348
in the spinal cord in CCI mice were not changed on the 349
contralateral side in comparison with naı¨ve mice. On the 350
ipsilateral side, mRNA level of all types of opioid 351
A
B
Fig. 1.The level of mechanical (A; von Frey test) and thermal (B;
cold plate test) hypersensitivity measured in naı¨ve (N) and 1, 14 and 28 days after injury in CCI mice. The data are presented as mean
± S.E.M. (9–15 mice per group). Intergroup differences were analyzed using Bonferroni’s multiple comparison tests.***p< 0.001 indicates a significant difference compared with the control (naive) animals; ooop< 0.001 indicates a significant difference compared with the contralateral side on the respective day after CCI.
352 receptors significantly decreased in the spinal cord
353 (Fig. 2A–C). The level of MOP, DOP and KOP receptor
354 mRNA in the thalamus was reduced significantly on the
355 contralateral side in comparison with naı¨ve mice. On the
356 ipsilateral side of the thalamus, only mRNA of MOP
357 receptor decreased (Fig. 2D). The levels of mRNA for
358 the DOP and KOP receptors did not change on the
359 ipsilateral side (Fig. 2E, F).
The level of PDYN and (PENK 360
mRNA in the spinal cord and 361
thalamus measured in naı¨ve 362
mice and 14 days after injury in 363
CCI mice 364
No changes in PDYN and PENK 365
mRNA levels were observed in 366
the contralateral part of the spinal 367
cord; in contrast the levels for 368
both PDYN and PENK mRNA in 369
ipsilateral part of the spinal cord 370
were significantly elevated 371
(Fig. 3A, B). PDYN and PENK 372
mRNA levels were not 373
significantly altered in either the 374
contra- or ipsilateral side of the 375
thalamus (Fig. 3C, D). 376
Dose-dependent stimulation of 377
GTPcS binding produced by 378
selective ligands of opioid 379
receptors in the spinal cord and 380
thalamus measured in naı¨ve 381
mice and 14 days after injury in 382
CCI mice 383
The observed decreases in 384
maximal stimulation (Emax) values 385
in membranes prepared from 386
naive animals were not significant 387
in any case. The samples from 388
CCI-exposed mice showed a 389
difference in G-protein activity 390
when comparing contra- and 391
ipsilateral sides. The maximal 392
stimulation was significantly lower 393
on the ipsilateral side in the spinal 394
cord, while a decrease was 395
observed on the contralateral side 396
in the thalamus for all three types 397
of opioid receptors (Table 1). 398
Significant differences between 399
contra- and ipsilateral sides were 400
found in CCI-exposed mice for 401
MOP receptors in the spinal cord 402
(***P= 0.0007; two-tailedP-value, 403
t= 9.594, df = 4) and in the 404
thalamus (**P= 0.0019; two-tailed 405
P-value, t= 7.306, df = 4). A 406
significant difference in G-protein 407
activation was observed when 408
comparing the contralateral and 409
ipsilateral sides of CCI-exposed 410
animals for DOP receptors in the spinal cord 411
(**P= 0.0031; two-tailed P-value, t= 6.402, df = 4) 412
and in the thalamus (*P= 0.0228; two-tailed P-value, 413
t= 3.598, df = 4). The effect was statistically significant 414
between the contralateral and ipsilateral sides of the 415
spinal cord (**P= 0.0012; two-tailedP-value, t= 8.284, 416
A D
E
F B
C
Fig. 2.qRT-PCR analysis of the MOP DOP and KOP mRNA levels in the both sides in naı¨ve mice (N) and ipsi- and contralateral parts of the spinal cord (A–C) and thalamus (D–F) 14 days after injury in CCI mice. The data are presented as mean ± S.E.M., which represent normalized averages derived from the threshold cycles obtained from qRT-PCR of 4–8 samples per group.*p< 0.05,**p< 0.01 and***p< 0.001 indicates a significant difference compared with the naive animals.
df = 4) and thalamus 417
(**P= 0.0011; two-tailed P-value, 418
t= 8.362, df = 4) in CCI-exposed 419
mice for KOP receptors (Table 1). 420
The GTPcS binding stimulated 421
by DAMGO, selective peptide 422
agonist of the MOP-receptor, was 423
similar in naı¨ve mice (both parts) 424
and the contralateral part of the 425
spinal cord in CCI-subjected mice, 426
yet it was much weaker on the 427
ipsilateral part of CCI-subjected 428
mice (Fig. 4A). The GTPcS 429
binding stimulated by 430
Ile5,6deltorphin II was very low but 431
similar between both parts in 432
naı¨ve mice and contra part of the 433
spinal cord in CCI mice, while 434
being slightly weaker in the 435
ipsilateral part of the spinal cord in 436
CCI mice (Fig. 4B). The GTPcS 437
binding stimulated by dynorphin1– 438 13was similar in naı¨ve mice (both 439
parts) and on the contralateral 440
part of the spinal cord in CCI 441
mice, but it was much weaker in 442
the ipsilateral part of the spinal 443
cord in CCI mice (Fig. 4C). 444
In the thalamus, the GTPcS 445
binding stimulated by the MOP 446
receptor selective peptide agonist 447
ligand DAMGO was similar in 448
naı¨ve mice (both parts) and the 449
ipsilateral part in CCI mice but 450
was much weaker on the 451
contralateral part of CCI mice 452
(Fig. 4D). The GTPcS binding 453
stimulated by Ile5,6deltorphin II 454
was very low but similar between 455
both parts of the thalamus in 456
naı¨ve mice and ipsilateral parts in 457
CCI mice, while significantly 458
weaker stimulation was observed 459
A
B
C F
E D
Fig. 3.qRT-PCR analysis of the PDYN (A, C) and PENK (B, D) mRNA levels in naı¨ve mice (N) and in the ipsi- and contralateral spinal cord (A, B) and thalamus (C, D) 14 days after injury in CCI mice. The data are presented as mean ± S.E.M., which represent normalized averages derived from the threshold cycles obtained from qRT-PCR of 6–10 samples per group.*p< 0.05,**p< 0.01 indicates a significant difference compared with the naı¨ve animals.
Table 1.G-protein activation by the selective opioid peptide receptor agonists DAMGO, Ile5,6deltorphin II and dynorphin1–13in the spinal cord and thalamic membrane preparations of naive and CCI mice on the contra- and ipsilateral sides
Maximal stimulation (efficacy)Emax± S.E.M. (%)
Receptor Spinal cord Thalamus
Contra Ipsi Contra Ipsi
MOP Naive 147.5 ± 2.8 144.4 ± 2.1NS 151.1 ± 3.0 149.3 ± 3.2NS
CCI 137.0 ± 2.1 113.1 ± 1.3*** 126.7 ± 2.1 145.5 ± 1.5**
DOP Naive 117.3 ± 2.1 114.4 ± 1.0NS 125.1 ± 1.7 126.1 ± 0.7NS
CCI 116.1 ± 1.7 104.4 ± 0.6** 114.3 ± 2.9 125.9 ± 1.3*
KOP Naive 146.1 ± 1.6 141.6 ± 3.6NS 148.4 ± 2.3 151.1 ± 2.8NS
CCI 146.2 ± 1.0 125.2 ± 2.3** 126.5 ± 2.2 150.2 ± 1.8**
Experimental data were processed by GraphPad Prism 5.0 using the sigmoid fit option of the dose–response curves. NS: not significant;*p< 0.05,**p< 0.01 and
***p< 0.001 based on unpairedt-tests.
460 only on the contralateral thalamus in CCI mice (Fig. 4E).
461 The GTPcS binding stimulated by dynorphin1–13 was
462 similar in naı¨ve mice (both parts) and in the ipsilateral
463 part in CCI-subjected mice, but it was weaker on the
464 contralateral part of thalamus in CCI mice (Fig. 4F).
465 DISCUSSION
466 The present study assessed neuropathic pain-related
467 behavioral changes accompanied by dynamic and
468 specific alterations of opioid system gene expression
469 levels and opioid receptor activity in the nociceptive
470 neuronal structures, the spinal cord and thalamus. In
471 the spinal cord, opioid peptide gene expression levels
472 increased in the parts ipsilateral to the site of the injury.
473 These changes were accompanied by tactile
474 hypersensitivity that was most pronounced on day 14.
These increases in opioid peptide 475
gene expression may suggest the 476
enhancement of peptidergic 477
neuronal activity. Increase in the 478
synthesis of opioid prohormones 479
and the subsequent release of 480
endogenous ligands, 481
accompanied by a decrease in all 482
MOP, DOP, KOP opioid receptor 483
gene expression levels and 484
decreased functional activity of 485
these receptors, as examined by 486
GTPcS binding on day 14 (the 487
chosen time point, when the 488
strongest behavioral changes 489
were observed), in the ipsilateral 490
spinal cord and contralateral 491
thalamus in a mouse model of 492
neuropathic pain were 493
demonstrated. These results are 494
in agreement with other studies 495
that described significantly 496
decreased density of MOP 497
receptor immunoreactivity in the 498
dorsal horn of the spinal cord in a 499
rat model of neuropathic pain 500
(Kohno et al., 1999; Zo¨llner et al., 501
2003), suggesting a link to reduced 502
opioid analgesia. 503
The view is now accepted that 504
there is a loss in spinal opioid 505
responsiveness under neuropathy 506
(Cahill et al., 2003). It has been 507
shown that phosphorylated-MOP 508
receptor-like immunoreactivity is 509
increased on the ipsilateral side in 510
the superficial laminae of the L5 511
lumbar spinal dorsal horn after sci- 512
atic nerve-ligation in mice; the 513
authors conclude that this, at least 514
in part, contributes to the reduction 515
in the antinociceptive effect pro- 516
duced by morphine (Narita et al., 517
2004). Other studies have shown 518
significantly decreased density of 519
MOP receptor immunoreactivity in the dorsal horn of the 520
spinal cord in a rat model of neuropathic pain (Kohno 521
et al., 1999; Zo¨llner et al., 2003), suggesting a link to 522
reduced opioid analgesia. Opioids injected intrathecally 523
activate spinal pre- and postsynaptic opioid receptors, of 524
which 50 to 70% are presynaptically located on primary 525
afferents (Gouarde`res et al., 1991; Abbadie et al., 526
2002). Neuropathy induced by peripheral nerve injury 527
has been shown to cause profound reorganization of the 528
nociceptive circuits within the spinal cord and the brain, 529
including changes in gene expression and morphology 530
(Mayer et al., 1999; Ossipov et al., 2000; Przewlocki 531
and Przewlocka, 2001). However, the basis for the lack 532
of opioid efficacy remains unclear. In 1999, Kohno et al. 533
indicated that nerve damage negatively influences the 534
action of MOP receptor agonists; the pre- and postsynap- 535
B B
A C
D
Fig. 4.Opioid receptor signaling mediated by specific ligands in membranes prepared from spinal cord and thalamus of naı¨ve and CCI mice in ipsi- and contralateral side. The maximal efficacy (Emax) above the basal activity of MOP, DOP and KOP receptors in stimulating G-proteins in the spinal cord (A–C) and thalamus (D–F). Percent increases (%) in the specifically bound radiolabeled nucleotide [35S]GTPcS are given above the basal (taken to be 100%) activity as a function of increasing concentrations (1010–105M) of DAMGO, Ile5,6deltorfin II and dynorphin1–13, a MOP, DOP and KOP receptors ligand, respectively. Points represent mean value ± S.E.M. for three experiments performed in triplicate. The level of basal activity indicates constitutive G-protein activity in the absence of any stimulating ligand.
536 tic inhibition of excitatory postsynaptic currents, caused
537 normally by such agonists, is less effective under nerve
538 injury conditions. The hyperexcitability of spinal neurons
539 with unilateral changes in opioid system activity is trans-
540 mitted to the thalamus under neuropathic conditions.
541 The ventral posterior thalamus is the major termination
542 site for the spinothalamic tract, and it relays nociceptive
543 activity to the somatosensory cortex. During neuropathic
544 pain, changes in neuronal firing in characteristic groups
545 of neurons occur (Patel and Dickenson, 2016). Changes
546 in endogenous opioid system activity in this structure
547 may be very important for the final feeling of pain.
548 In recent years, several papers have identified an
549 asymmetric distribution of opioid receptors and their
550 endogenous ligands after traumatic brain injury
551 (Bakalkin et al., 1982; Bakalkin and Kobylyansky, 1989;
552 Bakalkin, 1989; Hussain et al., 2012). Unilateral changes
553 in opioid receptor binding were also observed in chronic
554 pain patients with central post-stroke pain, which was
555 considered to reflect a sustained increase in the release
556 of endogenous opioids. In those patients, interhemi-
557 spheric comparisons using positron-emission tomography
558 demonstrated a significant decrease in [11C]diprenorphine
559 binding in the posterior midbrain, medial thalamus and the
560 insular, temporal and prefrontal cortices contralateral to
561 the painful side (Maarrawi et al., 2007a,b). Our observa-
562 tion of MOP receptor changes in the spinal cord and tha-
563 lamus support and supplement the above information with
564 results in the animal model; furthermore, they extend this
565 clinical observation with information on changes in other
566 opioid receptors. In our study, KOP receptor mRNA levels
567 significantly decreased on day 14 in the ipsilateral part of
568 the spinal cord and contralateral part of the thalamus.
569 Interestingly, the study ofXu et al. (2004)indicates that,
570 in contrast to our results, KOP immunoreactivity was
571 markedly increased in the L4-L5 spinal dorsal horn of
572 C57BL/6 mice 7–21 days after injury but not in mice pre-
573 treated with the KOP antagonist nor-binaltorphimine
574 (norBNI). On the other hand, in 2003, we showed
575 (Obara et al., 2003) that the administration of KOP recep-
576 tor antagonists norBNI and 50-guanidinonaltrindole (GNTI)
577 enhanced pain in rats and mice in a CCI model of neuro-
578 pathic pain. The hypersensitivity potentiation after norBNI
579 or GNTI administration was inhibited by the earlier admin-
580 istration of dynorphin antibody or ketamine. Our results
581 suggest that enhanced sensitivity is mediated through
582 non-opioid effects of the endogenous opioid peptide,
583 dynorphin. The spinal release of PDYN-derived ligands
584 after nerve injury is known to contribute to neuropathic
585 pain development (Obara et al., 2003; Labombarda
586 et al., 2008; Mika et al., 2010; Chen et al., 2014;
587 Rojewska et al., 2014). Their non-opioid action is potenti-
588 ated by the blockade of KOP receptors; this finding corre-
589 sponds with the elevation of PDYN mRNA levels in the
590 ipsilateral part of the spinal cord in our experiments. In
591 addition, knock-out mice lacking PDYN, KOP, or G-
592 protein receptor kinase 3 did not show significant
593 increases in KOP immunoreactivity after spinal nerve liga-
594 tion. KOP knock-out mice developed significantly
595 increased tactile and thermal hypersensitivity in both the
596 early (first week) and late (third week) intervals after
injury. It has been suggested that endogenous dynorphin 597
has both pronociceptive and antinociceptive actions after 598
nerve injury (Xu et al., 2004; Rojewska et al., 2014). The 599
dynorphin also acted as an endogenous agonist at KOP 600
receptors. Numerous studies have documented the 601
antinociceptive effects of the intrathecal and systemic 602
administration of selective KOP agonists (Nakazawa 603
et al., 1991; Kolesnikov et al., 1996; Obara et al., 2003; 604
Rojewska et al., 2014). Thus, the endogenous opioids 605
derived from PDYN may have both antinociceptive and 606
pronociceptive actions. It is not clear how the sustained 607
activation of opioid receptors caused by endogenous 608
dynorphin contributes to the neuropathic pain state; as 609
the dynorphin level is higher in neuropathy than in physi- 610
ological conditions, it is probably that it may be able to 611
activate potentially pronociceptive receptors (such as 612
NMDA and bradykinin receptors) after the saturation of 613
KOP receptors (Vanderah et al., 1996; Obara et al., 614
2003; Rojewska et al., 2014). Furthermore, KOP receptor 615
functional activity is weaker, as was shown in our study 616
with GTPcS binding. The lower functional activity of 617
KOP receptors might shift the balance from antinocicep- 618
tive to pronociceptive actions of the endogenous dynor- 619
phin system and thus contribute to the weakening of the 620
effects of opioid drugs in neuropathic pain. 621
Changes in the DOP receptor mRNA expression show 622
lateralized and functional changes that differed depending 623
on the structure. DOP receptor mRNA level decreased in 624
the ipsilateral part of the spinal cord on the 14th day, while 625
in the thalamus, a decrease was observed in the same 626
time point but only on the contralateral side. The 627
changes in functional activity measured by GTPcS 628
binding showed differences depending on the structure. 629
In the thalamus, a potent contralateral decrease in 630
expression was accompanied by a very dynamic 631
difference in functional GTPcS binding to DOP receptors 632
in a wide range of doses. The described strong 633
contralateral changes in the thalamic pain pathways, 634
occurring in all opioid receptors in both their expression 635
and GTPcS binding, may reduce the effect of opioid 636
drugs in this kind of pain, but this aspect requires further 637
research. 638
In contrast, in the spinal cord, the significant decrease 639
in the ipsilateral level of DOP receptor mRNA was 640
accompanied by a slight, much less pronounced than 641
for MOP and KOP, decrease in GTPcS binding to this 642
receptor. Interestingly,Obara et al. (2009)used an ED50 643
analysis to demonstrate that much higher doses of MOP 644
and KOP agonists injected intraplantarly are required to 645
produce analgesia in neuropathic versus inflammatory 646
pain; in contrast, the ED50of DOP agonists is comparable 647
in both models of chronic pain. Many studies have shown 648
that selective DOP agonists do not lose their effective- 649
ness in neuropathic pain (Mika et al., 2001, 2014; 650
Gave´riaux-Ruff and Kieffer, 2011). 651
Our experiments show that changes in GTPcS binding 652
are similar between spinal and thalamic MOP/KOP 653
receptors, whereas the activation of DOP receptors is 654
remarkably different in both structures studied. In the 655
spinal cord, the difference in opioid ligand stimulation of 656
DOP receptors was minimal, while in the thalamus, the 657
658 binding level on the contralateral side dropped
659 significantly for a wide range of doses.
660 The spinal differences in GTPcS binding of MOP and
661 KOP receptors compared to DOP receptors are in
662 agreement with our previous behavioral studies. Mika
663 et al. (2014)showed that selective agonists of MOP and
664 KOP receptors (DAMGO and U50,488H, respectively),
665 in contrast to DOP receptor agonists (DPDPE, deltorphin
666 II or SNC80), lose their analgesic effectiveness after
667 nerve injury. Our preliminary data performed in the CCI
668 model on mice on day 14 after injury indicate a lower
669 ED50 for morphine given i.th. in comparison to naive ani-
670 mals (1.25 g vs 2.9 g, respectively), while the ED50 for
671 enkephalin is very close to the values obtained in naive
672 mice (0.03 g vs 0.05 g, respectively). We suggest that this
673 difference may be related to the fact that DOP analgesia
674 is not dependent on injury-induced microglial activation.
675 Ourin vitrostudy (Mika et al., 2014) confirmed the pres-
676 ence of MOP/KOP receptors and the concurrent absence
677 of DOP receptors in microglial cells. This is in agreement
678 with other studies that have shown that microglia express
679 MOP and KOP receptors (Chang et al. 1996; El-Hage
680 et al. 2013; Merighi et al. 2013).Chao et al. (1996) first
681 reported in 1996 that KOP receptors are present in
682 human microglia, and the expression was confirmed by
683 the membrane binding of the selective ligand [3H]
684 U69,593. Thus, a slight change in GTPcS binding of
685 DOP ligands in the mouse CCI model in our studies
686 may explain this lack of change in the analgesic response
687 of these ligands after their spinal or peripheral administra-
688 tion compared to the attenuated MOP and KOP effi-
689 ciency. However, in the thalamus, we demonstrated a
690 very strong reduction in the GTPcS binding of this recep-
691 tor. This change may be important in reducing the central
692 effect of opioid drugs that have an MOP/DOP activity pro-
693 file. The functional state of the MOP receptor is known to
694 be dependent on the DOP receptor (Scherrer et al.,
695 2009), and the strong weakening of the binding of these
696 two receptors in the structures important for the central
697 effects of opioid drugs can have a large impact on the
698 analgesic effect of opioid drugs in neuropathic pain.
699 SUMMARY
700 Activity of opioid systems is altered by neuropathy
701 development, which induces an increase in endogenous
702 opioid peptide availability, which consequently results in
703 transiently enhanced opioid receptor occupancy leading
704 to a likely decrease in receptor expression. Our studies
705 provide evidence for selective changes in the activity of
706 spinal and thalamic opioid systems in a mouse
707 neuropathic pain model. Our experiments show that
708 similar changes in the GTPcS binding of MOP and KOP
709 receptors occurred in the spinal cord and thalamus,
710 whereas the binding to the DOP receptor was very
711 different depending on the structure. At the spinal cord
712 level, the difference in ligand binding to the DOP
713 receptor was minimal which may explain the lack of
714 lower efficacy of DOP receptor ligands after their i.th. or
715 i.pl. administration in neuropathic pain model. However,
716 strong reduction in the thalamic GTPcS binding may be
the cause of reduced central effect of opioid drugs with 717
MOP/DOP efficacy in neuropathic pain. 718
UNCITED REFERENCE 719
Xanthos et al. (2011). 720
CONFLICT OF INTEREST 721
None to disclose. 722
ACKNOWLEDGMENTS 723
This study was supported by the National Science Centre, 724
Poland, grant MAESTRO 2012/06/A/NZ4/00028 and 725
statutory funds from the Institute of Pharmacology at the 726
Polish Academy of Sciences and European 727
Commission, FP7 (#HEALTH-F2-2013-602891); J.S. is 728
a holder of a KNOW scholarship sponsored by the 729
Ministry of Science and Higher Education, Poland. The 730
work (E.S. and S.B.) was supported by the Polish (PAN) 731
and Hungarian (MTA) Academy of Sciences, which 732
provided bilateral researcher exchange programmes. 733
REFERENCES 734
Abbadie C, Lombard MC, Besson JM, Trafton JA, Basbaum AI (2002) 735 Mu and delta opioid receptor-like immunoreactivity in the cervical 736 spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: 737 an analysis of pre- and postsynaptic receptor distributions. Brain 738 Res 15:150–162. 739
Albe-Fessard D, Berkley KJ, Kruger L, Ralston 3rd HJ, Willis Jr WD 740 (1985) Diencephalic mechanisms of pain sensation. Brain Res 741 356:217–296. 742
Bakalkin GYA (1989) Neuropeptides induce directional asymmetry in 743 brain and spinal cord: facts and hypotheses. Int J Neurosci 744 48:105–124. 745
Bakalkin GYA, Kobylyansky AG (1989) Opioids induce postural 746 asymmetry in spinal rat: the side of the flexed limb depends upon 747 the type of opioid agonist. Brain Res 20:277–289. 748
Bakalkin GYA, Krivosheev OG, Stolyarov GK (1982) Postural 749 asymmetry in rats induced by stress and pain stimuli. Life Sci 750 30:779–783. 751
Bencherif B, Fuchs PN, Sheth R, Dannals RF, Campbell JN, Frost JJ 752 (2002) Pain activation of human supraspinal opioid pathways as 753 demonstrated by [11C]-carfentanil and positron emission 754 tomography (PET). Pain 99:589–598. 755
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that 756 produces disorders of pain sensation like those seen in man. Pain 757 33:87–107. 758
Bradford MM (1976) Rapid and sensitive method for the quantitation 759 of microgram quantities of protein utilizing the principle of protein- 760 dye binding. Anal Biochem 72:248–254. 761
Cahill CM, Dray A, Coderre TJ (2003) Intrathecal nerve growth factor 762 restores opioid effectiveness in an animal model of neuropathic 763 pain. Neuropharmacology 45:543–552. 764
Chang AC, Chao CC, Takemori AE, Gekker G, Hu S, et al. (1996) 765 Arylacetamide-derived fluorescent probes: synthesis, biological 766 evaluation, and direct fluorescent labeling of kappa opioid 767 receptors in mouse microglial cells. J Med Chem 39:1729–1735. 768 Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu DF, Archer S, 769 Bidlack JM, Peterson PK (1996) Kappa opioid receptors in human 770 microglia downregulate human immunodeficiency virus 1 771 expression. Proc Natl Acad Sci USA 23:8051–8056. 772
Chen X, Wang T, Lin C, Chen B (2014) Effect of adenoviral delivery of 773 prodynorphin gene on experimental inflammatory pain induced by 774 formalin in rats. Int J Clin Exp Med 7:4877–4886. 775