A M O D E L F O R A C T I V E T R A N S P O R T
R I C H A R D H. W I L H E L M
Department of Chemical Engineering, Princeton University, Princeton, New Jersey
As a contribution to this interdisciplinary conference, I a m presenting t h e elements of p a r a m e t r i c p u m p i n g [ 1 2 ] , a m e a n s for s e p a r a t i n g liquid mixtures currently under s t u d y in our l a b o r a t o r y . T h e subject involves t r a n s p o r t considerations, a n d hence is suggested to lie within t h e scope of t h e meeting. I t provides a h a n d y illustration of m i n i - mum-essential m a t h e m a t i c a l model-building procedures t h a t underlie the engineering design of large-scale chemical p l a n t s . P e r h a p s more relevant to biological interest is t h e observation t h a t p a r a m e t r i c p u m p i n g is a form of active t r a n s p o r t , a process by which a mass flux against an a p p a r e n t l y adverse fluid-phase concentration gradient is developed in a localized s t r u c t u r e a t t h e expense of some form of energy. F u r t h e r m o r e , because of t h e m a t h e m a t i c a l n a t u r e of t h e model description, generalizations are possible, a n d applicability of the model m a y be explored beyond t h e i m m e d i a t e macroscopic s e p a r a - tion process for which it presently is written. Speculation t h u s is invited regarding t h e relevance (or lack of it) of t h e model to cellular t r a n s p o r t by t h e usual procedure of comparing system responses of t h e model with those in n a t u r e . This comparison is m a d e in t h e l a t t e r p a r t of t h e paper between ion t r a n s p o r t characteristics of giant squid axon cells, as determined experimentally by H o d g k i n and H u x l e y , a n d system properties of p a r a m e t r i c pumping.
B y p a r a m e t r i c p u m p i n g , generally, is m e a n t coupled action of one oscillatory field upon another to give a desired result. F o r example, two pendulums, whose weights are connected t h r o u g h a spring, exercise complex p a r a m e t r i c a l l y induced motions on each other. Laser action depends upon coupling of electromagnetic waves (light) with q u a n t u m mechanical electronic fields of atoms. I n t h e present instance, solid- liquid adsorptive equilibria are caused to be displaced in a cyclic fashion by a " p u m p " which itself is an oscillatory t h e r m a l field. T h e n e t consequence of t e m p e r a t u r e changes acting p a r a m e t r i c a l l y on t h e
199
solid-fluid composition fields is t h e formation of a limiting-value, t i m e - a v e r a g e , fluid-phase, spatial concentration gradient in a fixed bed of adsorptive particles. Such a gradient comprises t h e basis of a solute separation scheme a t t h e u l t i m a t e expense of t h e r m a l energy.
A significant separation b y this procedure has been achieved experi- m e n t a l l y for N a C l in water, for example.
T h e subject is developed in w h a t follows t h r o u g h these topics.
F i r s t , p a r a m e t r i c p u m p i n g is described operationally a n d is formulated m a t h e m a t i c a l l y for t h e macroscopic adsorption-separation system.
C e r t a i n q u a l i t a t i v e a n d q u a n t i t a t i v e properties of t h e system are dis- cussed. Next, generalizations of t h e p a r a m e t r i c p u m p i n g principle are suggested for possible application to structured systems regardless of u l t i m a t e component dimensions, n u m b e r of variables, or kinds of t h e r m o d y n a m i c driving forces. T h i r d a n d finally, numerical solutions of t h e system of coupled equations are presented as gross analogs of classic voltage-clamp nerve cell experiments. Some points of possi- ble consistency between t h e cited experiments and a p a r a m e t r i c p u m p - ing model for active diffusion are discussed. R e s u l t s encourage sugges- tions for experimental work to detect t h e presence or absence of fine- scale oscillatory phenomena during t h e course of active t r a n s p o r t , together with suggestions for further theoretical development.
P A R A M E T R I C P U M P I N G Operational Description
Application of d y n a m i c adsorption principles for separating t h e components of a homogeneous b i n a r y liquid m i x t u r e is illustrated by m e a n s of t h e experimental elements depicted in Fig. 1. A column containing a bed of porous, p a r t i c u l a t e , adsorptive m a t e r i a l and a charge of the mixture is equipped a t its ends by driving and driven pistons acting in t a n d e m . T h e pistons cause relative position displace- ments to t a k e place between the column of particles and t h e column of a m b i e n t fluid. As p o r t r a y e d , t h e system is closed and its t o t a l volume is constant. Initially, let each phase be uniform in solute concentration, let the t e m p e r a t u r e be uniform t h r o u g h o u t t h e column a n d p e r m i t a solute concentration equilibrium to become established between liquid and solid phases. T h e r e u p o n a t a s t a r t i n g time, £Q. a t h e r m o d y n a m i c gradient is imposed on t h e column, as, for example, by bringing fluid t e m p e r a t u r e s a t t h e column ends to different constant values t h r o u g h t h e use of heat sources and sinks. I t has been shown
experimentally a n d theoretically t h a t after t h e above n o n s y m m e t r i c a l process a r r a n g e m e n t h a s been initiated a n d continued to limiting con- ditions (i.e., until t i m e - a v e r a g e d values of all properties h a v e become c o n s t a n t ) , a n axial fluid-phase, t i m e - a v e r a g e , solute composition g r a d i e n t will h a v e developed. T h e resulting difference in interparticle solute concentration a t the column ends, which arises from coupled
θ5, T E M P E R A T U R E OF P A R T I C L E S
φ8, S O L U T E COMPOSITION IN P A R T I C L E S θ , T E M P E R A T U R E OF
I N T E R S T I T I A L FLUID φ , S O L U T E C O M P O S I T I O N
IN I N T E R S T I T I A L F L U I D
n m
FIG. 1. Experimental arrangement for closed-system separation by means of thermal parametric pumping.
h e a t a n d m a s s transfer processes within t h e bed, is t h e separation in question.
I n common with other separation processes, individual columns m a y be used as b a t c h separators w i t h t o t a l reflux, as shown in Fig.
1, as continuous-flow, open-system, single columns with v a r i o u s a r - r a n g e m e n t s for feed introduction a n d p r o d u c t w i t h d r a w a l a n d finally as elements in multicolumn a r r a y s ; different system a r r a n g e m e n t s lead to a range of separation p o t e n t i a l s a n d t h e r m a l efficiencies.
B y w a y of further amplification, consider t h e following cycle:
a fluid volume arrives at a stroke-end level, raising the temperature of the adjacent adsorbent. As a result of temperature change, the adsorbent transfers solute to the fluid. Enriched (and cooled) fluid next is displaced axially to contact warmer adsorbent. The fluid cools the adsorbent now adjacent and loses solute to it. Transport of fluid back to the point of origin completes the cycle. The difference between the fluxes of solute in the two flow directions is the net flux due to parametric pumping. I n closed systems the net flux approaches zero as limiting conditions are reached.
A central concept of parametric pumping thus comprises designed alternating displacements from equilibrium of the solid-fluid composi- tions coupled with judicious timing of the axial flow displacements.
Mathematical Model
In this section, the differential equations and initial and boundary conditions for material separation by thermal parametric pumping are presented. The physical meaning of each term and its characteris- tic dimensionless coefficient are identified. (Dimensional nomenclature is presented elsewhere [12].) Essential features t h a t cause the equa- tions to have parametric pumping characteristics also are noted briefly.
The following five equations taken together with initial and bound- ary conditions comprise a system description :
Heat Balances
^ + y(es - Bf) = 0 (2)
Mass Balances
a Φ,
m + λ ( φ / * - Φ / ) = ο (4)
Equilibrium
φ / * = Β + Όθ3 + ς>φ8 + ϋφ,θ, + £ φ , 2
+ W + · · · ( δ ) Equations (1) and (2) are dimensionless heat balances in time and position for interparticle and intraparticle space, corresponding dimensionless temperatures being Bf and 0S (see Fig. 1). Dimension-
less t e m p e r a t u r e s are defined to v a r y between 0 a n d 1 within t h e t e m p e r a t u r e limits of t h e s y s t e m ; t a n d ζ are dimensionless t i m e and axial position variables.
E q u a t i o n s (3) a n d (4) are dimensionless mass balances, inter- particle a n d i n t r a p a r t i c l e concentrations being φ; a n d φ8 respectively.
T h e fact t h a t the mass balances are here written for only one com- ponent assumes t h a t solute is present in dilute concentration. I n general, there would be η m a s s balances for η components.
T h e first t e r m of E q . (1) is t h e axial fluid-phase convective h e a t - transfer r a t e , t h e second t e r m is t h e fluid-phase t r a n s i e n t , t h e third, the solid-phase t r a n s i e n t , and t h e fourth, an effective axial t h e r m a l diffusivity. T h e two t e m p e r a t u r e variables are linked t h r o u g h E q .
( 2 ) , which is a simplified r a t e equation for transfer of h e a t to and from particles. T e r m s in E q s . (3) a n d (4) h a v e meanings analogous to those in E q s . (1) and ( 2 ) .
B o t h t h e r m a l a n d compositional field e q u a t i o n s are " d r i v e n " b y t h e periodic velocity, af(t). I t is essential t h a t this driving coefficient of t h e d e r i v a t i v e d/dz change in sign periodically.
Coupling between the set of t h e r m a l fields [ E q s . (1) and ( 2 ) ] a n d the set of compositional fields [Eqs. (3) and ( 4 ) ] is accomplished through t h e equilibrium relationship [ E q . ( 5 ) ] . N o t e t h a t t h e equilib- rium fluid-phase composition, φ/*, is a function of both φ8 a n d θ8, t h e composition a n d t e m p e r a t u r e , respectively, of t h e local solid phase.
T h e " p u m p " is identified as the oscillatory t h e r m a l field. T h e larger the positive contribution to the equilibrium fluid composition, φ,*, of the terms containing the solids t e m p e r a t u r e , θ8, the larger will be t h e potential separation. N o n l i n e a r i t y in t h e equilibrium relation m a y contribute positively to the extent of separation b u t t h e fact of sep- aration does not seem to depend on it. However, in p a r a m e t r i c proc- esses a nonlinearity developed through t h e coupling action between fields can lead to amplifications in the system o u t p u t . I n t h e present instance t h e generation of nonlinearity (higher harmonics) through the first terms of E q s . (1) and (3) can lead, for selected combinations of system p a r a m e t e r s , to very substantial amplifications of concentra- tion ratios over a column length.
Separation as well as t h e r m a l efficiencies depend upon these dimen- sionless coefficients :
af(t) Velocity of fluid. F o r a given fit), a d e t e r m i n e s t h e d i s p l a c e m e n t a m p l i t u d e , which m a y be larger or smaller t h a n t h e column l e n g t h .
1 / 7
1/λ
R a t i o of t h e a v e r a g e v o l u m e t r i c h e a t c a p a c i t y of t h e a d s o r b e n t p h a s e t o t h a t of t h e fluid p h a s e . R a t i o of v o l u m e w i t h i n t h e solid a d s o r p t i v e p h a s e t o t h a t of t h e fluid p h a s e .
T i m e c o n s t a n t for t h e r m a l response of a d s o r p t i v e particles relative t o t h e characteristic t i m e of d i s p l a c e m e n t .
T i m e c o n s t a n t for m a s s transfer response of a d s o r p - t i v e particles c o m p a r e d t o t h e characteristic t i m e of d i s p l a c e m e n t .
D i s s i p a t i v e axial diffusivity of h e a t . D i s s i p a t i v e axial diffusivity of solute m a s s . E m p i r i c a l c o n s t a n t s in equilibrium E q . (5).
B, D, Q, R S, U
B o u n d a r y and initial conditions for v a r i o u s column a n d cascade a r r a n g e m e n t s differ in detail. F l u x of mass entering a column during one half cycle generally will depend upon t h e o u t p u t during t h e p r e - vious half cycle. F l u x of heat, in t u r n , depends upon the constant t e m - p e r a t u r e imposed a t a column end a n d the inward flow of fluid a t t h a t end. Conditions applicable t o t h e closed system illustrated in Fig. 1, and used in solving t h e differential equations to secure figures pre- sented in the last section of t h e paper, are t h e s e :
Boundary Conditions SH(t,0) = 0
for vtt < t < 0 +
r
l) 7 r , ν = 0, 2, 4 . . .
ξ#(ί,1) = a sin t
for vir < t < (ν + 1)π, ν = 1, 3, 5 . . . u"dt"~\ . t / sin t
for νπ < t < (v + l ) x
0 - l)ir < t" < νπ, ν 'ίφ
1
sin t" dt"
0, 2, 4, 6 . . α sin t
/ sin t" dt"
for
vtt
< t < (v + l ) x(ν -
1)t
< t" <vtt,
ν = 1, 3, 5 . . . Initial Conditions (Typical)θΛΟ,ζ) = 0.(0,2) = e/t = ΘΒ,
φ/(0,«) = Β + ΏΘΗ + Q<t>H + R4,.ft„ + . ? φ8 ο 2
+ Ζ/θ,* + Φ,(0,ζ) = ΦΗ
(6) (7)
(8)
(9)
(10)
( Π )
(12)
T h e dimensionless s y m b o l s h a v e these m e a n i n g s :
£ i f H e a t a n d m a s s fluxes, respectively, i n t o column e n d s ; (t,0) for all times a n d cold end, (t,l) for all t i m e s a n d w a r m end.
ν Positioning index for sine w a v e fluid velocity, χ M a t h e m a t i c a l pi.
E x p e r i m e n t a l separations of N a d solutions h a v e been achieved with mixed-bed, ion-exchange resin in beds of t h e order of 18 inches high with a t e m p e r a t u r e difference of 5 0 ° C over t h e columns. Open- and closed-system operation has been performed; separation r a t i o s a t column ends were of the order of 1.1 to 1.2; cycle times were of t h e order of m i n u t e s .
Analog a n d digital solutions have been performed in s u p p o r t of experimentation a n d for exploration of model characteristics. As a result of these numerical solutions the following s t a t e m e n t s m a y be m a d e :
I n a p r o g r a m of model verification, initial agreement between ex- p e r i m e n t a n d t h e o r y is close.
Assuming only a previously measured equilibrium relationship [Eq. (5) ] for N a C l - r e s i n , other system constants also being in t h e experimental range, digital calculations yielded column-end s e p a r a - tion ratios of t h e order of 1.1 to 3.0. However, when t h e values of γ and λ, especially, were placed within a selected range, concentration multiplication was computed to t a k e place, separation ratios s u b - s t a n t i a l l y larger t h a n two orders of m a g n i t u d e being indicated as possibilities.
I n still other explorations with a different equilibrium relationship, separations of as m u c h as 28 were encountered c o m p u t a t i o n a l l y .
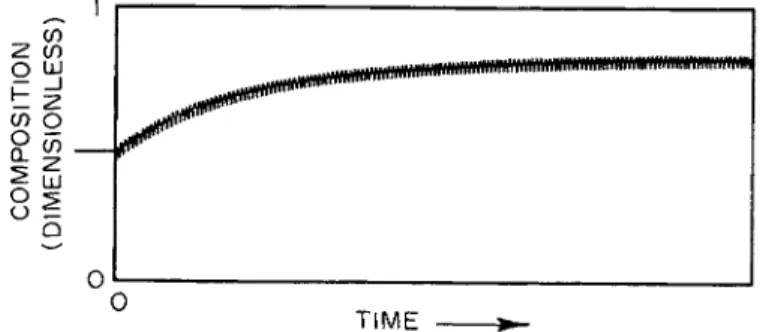
P a r a m e t r i c p u m p i n g exhibits t h e response t i m e characteristic of p a r a m e t r i c processes generally. S t a r t i n g from some initial condition the m e a n values of t h e dependent variables change deliberately until a long-time limiting condition has been achieved. Such a system t r a n s i e n t is illustrated in Fig. 2, which presents a composition r e a d o u t on a n analog computer simulation of t h e present process. T h e location for t h e r e a d o u t was chosen a r b i t r a r i l y to be n e a r one end of t h e column. M a i n t e n a n c e of separation depends upon m a i n t e n a n c e of
φ
1 Φ(1",0)
Some System Properties
oscillations everywhere continuously within the domain described by t h e differential equations.
I t is essential t h a t separation a t limiting conditions be finite. T h a t such is t h e case h a s been verified by computation for several a l t e r n a t e open a n d closed process a r r a n g e m e n t s . A l t e r n a t e initial conditions were arranged such t h a t t h e t r a j e c t o r y of a p p r o a c h to t h e limiting
T I M E
FIG. 2. Dependent variable-time trace characteristic of parametric pumping operation after process initiation or change in conditions. Curve secured at arbitrarily selected system site during analog simulation of Eqs. (1-5).
separation factor (as in Fig. 2) was approached from above and below its final v a l u e .
G EN ERA LI ZATION S
T h e general idea of coupling m a y be summarized in t h e following equations :
+ h
(13)^ = mx + η (14) χ and y are variables and a, b, m, and η are coefficients, some of
which are driven in an oscillatory fashion. Cross coupling between t h e variables in both directions is noted in this instance. N u m e r o u s examples of coupled processes with a single dependent variable (time or position) are e x t a n t [10] in electrical engineering or physics. Gen- erally, energetic processes involving electricity or m o v e m e n t of masses are involved and t h e conservation equations of motion and energy are called for. I n the present instance, t h e equations are mass and h e a t conservation balances and the corresponding fields are " d r i v e n "
simultaneously in a l t e r n a t i n g fashion in time by means of t h e f(t).
T h e f(t) in question p l a y s t h e same p a r t as an a l t e r n a t i n g current source in an electrical coupled process b u t here t h e velocity a l t e r n a - tions serve, t h r o u g h flow displacements between fluid a n d solid phases, simply to keep t h e system in a s t a t e of disequilibrium. P e r se, f(t) is not a direct energy source; this is supplied by p l a c e m e n t of h e a t sources a n d sinks a t the column ends. I n contrast to t h e set of coupled equations [Eqs. (13) and ( 1 4 ) ] , t h e present set [ E q s . ( 1 - 5 ) ] are coupled only in one direction; t h e oscillatory t h e r m a l field (in Θ) serves to " p u m p " the mass field (in φ) b u t t h e mass field does not affect the t h e r m a l field.
A v i r t u e of a system of equations t h a t comprise a model describing one physical situation is t h a t such a system, or a modification of it, m a y a t times be applied to completely different physical situations.
I n such analogous uses, it is essential only t h a t the basic structure or idea behind t h e system in the first place be r e t a i n e d ; in present work, t h e idea is t h a t a diffusion-flow system, driven in an oscillatory m a n n e r , a n d to which an energy source is applied, m a y lead to compo- sitional separation. T h e following comments are offered regarding gen- eralized application of E q s . (1-5) a n d their extensions.
T h e equations are independent of t h e scale of action. I n adsorber- separation, p a r t i c l e - s t r u c t u r e dimensions are of t h e order of a milli- meter, system dimensions are of t h e order of a foot, a n d t i m e con- s t a n t s , of a m i n u t e . W i t h a new set of coefficients a p p r o p r i a t e to t h e situation, t h e equations could be applied equally well t o a system having independent variables of the order, say of a h u n d r e d Angstroms and small fractions of a second.
T h e n u m b e r of dependent variables m a y be two or more and t h e physical interpretations placed upon t h e m m a y differ widely from application to application. T h u s , currently there are two t y p e s of dependent variables, θ and φ, representing t e m p e r a t u r e and composi- tion fields. T h e n u m b e r of such t y p e s of fields m a y be increased in- definitely as long as there are coupling relations, such as E q . ( 5 ) , operative between t h e m . D e p e n d e n t relations might include electrical, magnetic, chemical potential such as p H and similar fields, a field also being added for each component in a multicomponent system.
An essential r e q u i r e m e n t for p a r a m e t r i c p u m p i n g t o occur is t h a t the operation represented by f(t), which drives t h e system, a l t e r n a t e a b o u t zero, t h e r e b y changing sign in t h e process. T h u s , in t h e r m a l p a r a m e t r i c pumping, liquid flows b a c k a n d forth relative to a bed of particles, a n d in electrical applications, an a l t e r n a t i n g current is
applied. P a r e n t h e t i c a l l y , d e p e n d e n t v a r i a b l e s such as θ/, θ8, φ/, φ s) will oscillate a b o u t m e a n , finite values of t h e v a r i a b l e ; t h e f(t) driving v a r i a b l e , b y contrast, m u s t a l t e r n a t e a b o u t a m e a n v a l u e of zero. Also, in present equations, note t h a t t h e driving f(t)'s are identical for t h e r m a l a n d m a s s fields. T h i s m e a n s simply t h a t flow displacements a c t simultaneously to affect t h e processes b y which h e a t a n d m a s s are conveyed b a c k a n d forth axially. I n general, / ( £ ) ' s for different fields m a y be functions of each other, i.e., t h e y m a y be phase (in a n electrical sense) displaced, provided t h a t n e t phase
β
HIGH V A L U E O F 9
f IS
B O U N D A R Y C O N D I T I O N A T ONE S Y S T E M T E R M I N A L
©
LOW V A L U E O F Bf IS BOUNDARY C O N D I T I O N A T O T H E R S Y S T E M T E R M I N A L
E X T E R N A L L Y - G E N E R A T E D A L T E R N A T I N G f ( t )
J2_
0f , 0S = t ( z , t )
3 ©
C O U P L I N G F U N C T I O N
M E A N H I G H V A L U E O F
$f S U S T A I N E D A T O N E S Y S T E M T E R M I N A L
M E A N LOW V A L U E O F
<f>
f S U S T A I N E D A T O T H E R S Y S T E M T E R M I N A LFIG. 3. Generalized function flow sheet showing the essential elements (differ- ential equations, coupling function, driving function) of the model described by Eqs. (1-5).
relations between all system v a r i a b l e s still p e r m i t desirable p a r a m e t - ric results to occur.
T h e a l t e r n a t i n g function, /(£)> describes an action (flow) of one p h a s e (liquid) r e l a t i v e to a n o t h e r (bed of p a r t i c l e s ) . Although our experimental w o r k h a s been with columns of particles which are fixed in l a b o r a t o r y space, t h e r e is nothing in t h e differential e q u a t i o n s t h a t requires t h e solid p h a s e to be s t a t i o n a r y ; all t h a t is required is t h a t t h e r e be a relative displacement between fluid a n d solid phases.
F i n a l l y , coefficients on t e r m s (such as γ, λ) which are now con- sidered to be c o n s t a n t m a y be t h e driven coefficients. B o u n d a r y con- ditions also m a y be oscillatory.
F i g u r e 3 presents a generalized function flow sheet for the p a r a -
metric p u m p i n g equations given earlier in t h e p a p e r . T h e boxes show t h e relations between differential equations (boxes 1 and 2 ) , t h e cou- pling function ( 4 ) , t h e driving f(t) ( 3 ) , t h e b o u n d a r y conditions (5, 6 ) , a n d t h e results of solving t h e set of equations, i.e., t h e development of a sustained separation in the dependent variable, <j>f) a t the t e r m i - nals of t h e system (boxes 7 a n d 8 ) .
ACTIVE T R A N S P O R T
I n this section we speculate on t h e system structure of p a r a m e t r i c pumping, a macroscopic t y p e of active t r a n s p o r t , and its possible rele- vance to active t r a n s p o r t in biological cells. F i r s t a t e n t a t i v e flowsheet for a p a r a m e t r i c active t r a n s p o r t model is presented a n d its compo- nents are discussed. Second, comparison is m a d e between experimental findings of H o d g k i n a n d H u x l e y on giant squid axon cells a n d r e - sponses typical of a system of p a r a m e t r i c p u m p equations. Specifi- cally, several curve shapes secured by numerical solutions of E q s .
(1-5) are compared with experimental curves. Voltage clamp experi- m e n t s as well as direct active diffusion experiments and their time constants are touched upon. F i n a l l y , certain new biological experiments are suggested which m i g h t serve to determine w h e t h e r an oscillatory t r a n s p o r t process, such as is being discussed here, m a y or m a y n o t be involved in t r a n s p o r t a t t h e cellular level.
Parametric Pumping—Active Transport Model
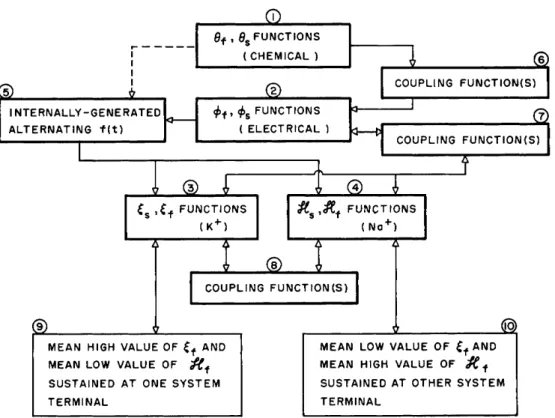
Figure 4 presents in flow sheet form three t y p e s of m a t h e m a t i c a l elements t h a t are essential to p a r a m e t r i c p u m p i n g ; differential e q u a - tions (boxes 1, 2, 3, 4 ) , coupling functions (boxes 6, 7, 8 ) , a n d an a l t e r n a t i n g driving function (box 5 ) . T h e n u m b e r of such boxes is chosen to p e r m i t inclusion in t h e framework of ideas frequently asso- ciated with biological active diffusion; degrees of freedom in a physi- cochemical sense m a y require more differential equations t h a n those suggested to close a n y m a t h e m a t i c a l formulation. I n c o n t r a s t to Fig.
3, no b o u n d a r y conditions are here indicated because t h e s t a t e m e n t of such conditions is possible only after t h e a p p r o p r i a t e differential equations h a v e been expressed in detail.
Consider t h e set of differential equations first. Box 1 refers to differential equations of metabolism chemistry, and hence t o t h e u l t i - m a t e source of e n e r g y ; box 2 refers to developed electrical p o t e n t i a l s such as h a v e been measured across t h e m e m b r a n e s ; a n d finally, boxes 3 and 4 describe the adsorptive interactions of N a
+ and K
+
in s t r u c -
0f , 0S F U N C T I O N S ( C H E M I C A L )
I N T E R N A L L Y - G E N E R A T E D A L T E R N A T I N G f ( t )
<£f, φ8 F U N C T I O N S ( E L E C T R I C A L ) I3
C O U P L I N G F U N C T I O N ( S )
J8L-L
£c F U N C T I O N S ( K
+
)
— 5 —
®
I
© A
C O U P L I N G F U N C T I O N ( S )
Z3
' ^ t
F U N
C T I O N S ( N a
+
)
®
C O U P L I N G F U N C T I O N ( S )
M E A N H I G H V A L U E O F £f A N D
M E A N LOW V A L U E O F #f
S U S T A I N E D A T O N E S Y S T E M T E R M I N A L
M E A N LOW V A L U E O F £f A N D
M E A N H I G H V A L U E O F ϋf
S U S T A I N E D A T O T H E R S Y S T E M T E R M I N A L
FIG. 4. Tentative generalized function flow sheet for active transport showing relationships between the essential elements : differential equations, coupling functions and driving function.
D H. WILHELM
t u r e d media such as cell walls, mitochondria a n d t h e like. A general s t a t e m e n t regarding t h e differential equations seems a p p r o p r i a t e a t this point. Chemical reactions a n d t h e energy released from t h e m , electrical potential a n d composition ( N a
+ , K
+
) , all are scalar q u a n t i - ties. T r a n s p o r t , on t h e other h a n d , is a vector q u a n t i t y . T o p e r m i t the desired coupling between t h e vector a n d scalar q u a n t i t i e s to occur requires (from t h e formulations of irreversible t h e r m o d y n a m i c s ) t h a t t h e l a t t e r q u a n t i t i e s h a v e a directional (anisotropic) sense. T h i s m e a n s simply t h a t t h e differential equations should contain space derivatives, whether these a p p l y to an adsorption column or to a cell wall.
R e t u r n i n g now to individual dependent v a r i a b l e s one a t a t i m e , a n d s t a r t i n g with the differential equations (boxes 1, 2, 3, 4 ; chemical reaction, electrical-potential, chemical composition v a r i a b l e s ) , let us proceed t o w a r d p l a c e m e n t of biological ideas into t h e framework.
Box 1 is intended to include all metabolism-associated e n z y m a t i c chemical steps which, according to recent concepts [1] supply energy for N a
+ a n d K
+
separation t h r o u g h an energy-rich p h o s p h a t e or p h o s - phagen. T h e over-all r a t e of a queue of sequential reaction steps is likely to be d y n a m i c a l l y of v e r y high order if t h e s u b s t r a t e s m u s t t r a v e l from one enzyme site to t h e next by diffusion, i.e., by a linear, irreversible process. F o r example, t h e oxidative p a t h w a y for glucose to oxaloacetate is commonly represented by thirteen direct steps
(neglecting side p a t h s ) . Considering t h e diffusive steps alone, t h e p r i n - ciples of control t h e o r y t e a c h t h a t in such a set of coupled steps t h e over-all r a t e b y which glucose is converted t o oxaloacetate is a t h i r t e e n t h - o r d e r equation. T o t h e extent t h a t t h e e n z y m a t i c reactions t h a t occur between successive diffusive steps are not a t equilibrium, t h e over-all order would be raised even more.
Chemical potential 0/,0
s functions (such as p H ) m a y couple di- rectly with the N a
+ - K
+
separation functions (electrical p o t e n t i a l in this instance being merely an evidence of separation a c c o m p l i s h m e n t ) . Alternatively, t h e chemical driving forces m a y a c t through electrical potentials as intermediates t o cause s e p a r a t i o n ; t h e l a t t e r course h a s quite a r b i t r a r i l y been depicted in Fig. 4.
Although measured electrical potentials across cell m e m b r a n e s are only of t h e order of 50 m v , structure thicknesses are small a n d local voltage gradients m a y be of t h e order of 100,000 to 150,000 volts
per centimeter. T h e influence of such a gradient on adsorptive equi- libria of charged species m a y be l a r g e ; box 2 is included in t h e flow sheet to represent t h e differential equations t h a t control t h e spatial and t e m p o r a l distribution of electrical charges.
Boxes 3 and 4 represent m a t e r i a l balance equations for charged N a
+ and K
+
species in c o u n t e r p a r t to E q s . (3) and (4) of this paper.
T h e n u m b e r of such sets of balances m a y be more t h a n t h e two represented in Fig. 4. F o r example, balances on other species such as anions a n d w a t e r also m u s t be included in principle if t h e concen- t r a t i o n s of these substances enter t h e local equilibria and their values oscillate significantly with t i m e .
Coupling functions indicated in boxes 6, 7, 8 are suggested to be c o u n t e r p a r t s to E q . 5 of t h e r m a l p a r a m e t r i c p u m p i n g . T h e chemical- electrical coupling of box 6 appears to have received little direct bio- logical experimental attention. However, for purposes of numerical exploration, m a t h e m a t i c a l s t a t e m e n t s regarding some elementary mechanism ideas m a y be introduced a t this point w i t h o u t destroying t h e over-all n a t u r e of t h e s y s t e m responses. On t h e other hand, for a sense of possible structure of t h e next coupling function, i.e., between electrical a n d compositional fields (box 7) one m a y d r a w upon t h e work of H o d g k i n a n d K a t z [ 6 ] , who related ionic composition in nerve cells a n d m e a s u r e d m e m b r a n e voltages to t h e N e r n s t equation, of E i s e n m a n [ 2 ] , who theoretically investigated t h e adsorptive selec- t i v i t y of ionic species as affected b y local, i n t e r n a l electrical field strengths, a n d of Kurella [ 9 ] , who measured voltages in ion-exchange resin model systems.
R e g a r d i n g couplings between composition fields (box 8 ) , t h e work of Helfferich [3] is suggested as relevant. H e has dealt with the complex exchange equilibria of ionized species on ionized resins. T h e couplings m u s t of course allow for opposed N a
+ a n d K
+
t r a n s p o r t flux directions such as occur in cells. I n this connection it is interesting t o note t h a t in t h e single-component p a r a m e t r i c p u m p i n g of N a C l on monobed ion exchange resins, t h e higher concentration of N a C l is found experimentally to congregate a t the upper, hotter end of the column. B y changing values of coefficients in the equilibrium rela- tion [ E q . ( 5 ) ] , a n d hence, in principle, changing t h e adsorber c h a r a c - teristics, it is found theoretically, t h r o u g h a numerical solution of
the model equations, t h a t N a C l can be caused to m i g r a t e t o w a r d t h e opposite, colder end of t h e column.
C e n t r a l l y i m p o r t a n t to separation b y p a r a m e t r i c operation is t h e a l t e r n a t i n g driving function, / ( £ ) , located in box 5 of t h e flow sheet.
Recall from earlier discussion t h a t f(t) m u s t a l t e r n a t e a b o u t zero, changing sign periodically. I n t h e r m a l p a r a m e t r i c p u m p i n g [Eqs.
( 1 - 5 ) ] a n d its generalization (Fig. 3 ) , note t h a t t h e driving function is externally generated by a l t e r n a t i n g flows of liquid b a c k a n d forth relative to a bed of resin particles. I n t h e t e n t a t i v e active t r a n s p o r t structure (Fig. 4 ) , t h e driving function is indicated to be internally generated as indeed it m u s t be if p a r a m e t r i c p u m p i n g p l a y s a p a r t in such t r a n s p o r t in biological cells. T h e provocative a n d highly inter- esting studies of Teorell [11] on a l t e r n a t i n g liquid flows induced by a m e m b r a n e - e l e c t r o l y t e s y s t e m u n d e r a dc voltage gradient would seem to provide a n a t u r a l , m a t h e m a t i c a l l y expressed c a n d i d a t e m e c h a - nism for f(t) of box 5. Although the t i m e c o n s t a n t for flow a l t e r n a t i o n in TeorelPs macroscopic model experiments are of t h e order of 30 minutes, scaling to the dimensions of cell m e m b r a n e s is likely to give time constants of very small fractions of a second, as p r o b a b l y would be called for if p a r a m e t r i c p u m p i n g were operative.
A p a r a m e t r i c p u m p i n g model, such as is suggested in Fig. 4, h a s some interesting over-all response properties t h a t , q u a l i t a t i v e l y a t least, do n o t depend upon t h e details of t h e functions in t h e boxes b u t r a t h e r on t h e fact of the coupling of t h e several layers of differen- tial equations a n d t h e driving of t h e m , externally or internally, by an / ( £ ) · T h u s , a change induced in one p a r t of t h e s t r u c t u r e m a k e s itself felt " d o w n s t r e a m " in t h e flow sheet of t h e coupled system. I n this sense p a r a m e t r i c p u m p i n g would seem to be consistent with t h r e e major t y p e s of t r a n s p o r t - r e l a t e d biological experiments. Interference with cellular m e t a b o l i s m t h r o u g h t e m p o r a r y or p e r m a n e n t metabolic poisons, or widely v a r y i n g t h e oxygen supply (box 1) induces changes in electrical p o t e n t i a l a n d in N a
+ a n d K
+
concentration differences.
I n voltage-clamp experiments, t h e s y s t e m is p e r t u r b e d a t t h e position of box 2 a n d t h e consequences a r e felt in changed ion concentration differences. F i n a l l y , in a classic t y p e of experiment, ion concentration of t h e same or a l t e r n a t e species is changed inside o r outside of cells or across m e m b r a n e s , a n d t h e consequences are observed t h r o u g h changes in ion concentrations elsewhere in t h e system or t h r o u g h changes in N e r n s t voltage across t h e system.
Comparison of Parametric Pump Solutions with Squid Axon Experiments
Classic voltage clamp a n d active ion diffusion experiments of H o d g k i n , H u x l e y , K e y n e s , a n d K a t z serve as a basis of comparison for theory. Consider first t h e d a t a (Fig. 5) of H o d g k i n [4] and H o d g - k i n a n d H u x l e y [5, 8] for potassium conductance against time com- p u t e d from a l t e r n a t i n g step-up a n d step-down voltage clamp experi- m e n t s . T h e a u t h o r s interpret t h e results in terms of m e m b r a n e per- m e a b i l i t y change and fit an empirical function to t h e curves as p a r t of t h e larger development of t h e ionic basis of nerve conduction.
: !
A
J>
ο ο „1 j\ Β
1 1 I 1 1
~ Ί
I 1 1 1 1 1
0 1 2 3 4 5 0 1 2 3 4 5
TIME ( M S E C ) TIME ( M S E C )
FIG. 5. Voltage clamp experiments on squid axon cells by Hodgkin and Huxley [5] expressed as potassium conductance versus time.
E q u a t i o n s a n d b o u n d a r y conditions (1-9) were solved using coeffi- cients conveniently a t h a n d from our macroscopic separation studies.
( I t is assumed t h a t t h e basic structure of t h e equations give q u a l i t a - tive curve forms t h a t do not differ in general shape as coeffici3nts are changed from one kind of problem to another.) Figure 6 presents results of one such calculation; separation r a t i o over t h e column is related t o n u m b e r of cycles (time) as axial diffusitivities, ψ a n d η, a r e varied simultaneously fourfold u p and down in a step-function fashion (actually accomplished b y changing t h e n u m b e r of axial com- p u t e r stages in t h e calculations). F i n a l distributions of values of all dependent variables a t t h e end of one computation are t a k e n as the initial conditions for the next j u m p calculation. ( T h e initial conditions t h a t are a r b i t r a r i l y imposed to get s t a r t e d on calculation are wiped o u t after one such step-function calculation.) Also, changing system
coefficients such as γ and λ, for example, between c o m p u t a t i o n cycles served to change only t h e m a g n i t u d e of abscissa and ordinate values, b u t n o t t h e characteristic curve shapes.
Comparison of Figs. 5 a n d 6 reveals a gross similarity in t h e t i m e course between experiment a n d model. C o m p u t e d results display t h e slowly rising, deliberate t r a n s i e n t s characteristic of p a r a m e t r i c processes a n d previously shown in Fig. 2. I n t h e present calculations t h e local oscillations, which are a l w a y s present, were purposely aver- aged out to save machine time. Also of interest is t h e fact t h a t rising a n d falling curves are superimposable, a necessary outcome of t h e fact t h a t E q s . (1-4) are linear a n d E q . 5 was linearized locally for purposes of t h e calculation. B y contrast, t h e squid axon d a t a are,
NUMBER OF C Y C L E S NUMBER OF C Y C L E S
( T I M E ) ( T I M E )
FIG. 6. Computed transient response in separation factor to alternating step function changes in axial diffusivity [φ and η in Eqs. (1 and 3 ) ] .
as t h e a u t h o r s pointed out, n o t superimposable and are highly non- linear. I t is clear t h a t the m a t h e m a t i c a l formulation would call for introduction of nonlinear components to describe t h e d a t a . Such might, for example, be a previously suggested nonlinear d y n a m i c response of t h e long sequence of first-order steps comprising t h e glycolysis chain. T h e n o n s y m m e t r y in t h e rising a n d falling curves of H o d g k i n a n d H u x l e y would p r o b a b l y also require t h e p a t h between chemical a n d electrical potential mechanisms, as is indicated in t h e coupling between boxes 1 a n d 2 in Fig. 4, to be one directional, as shown.
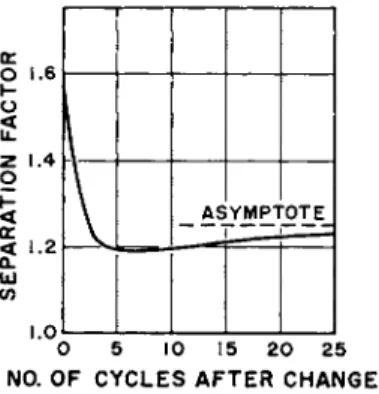
An interesting feature of p a r a m e t r i c p u m p i n g behavior is t h e achievement of a definite long-term, limiting v a l u e of all dependent, t i m e - a v e r a g e d variables. Limiting values of separation were explored c o m p u t a t i o n a l l y for a r a n g e of stage n u m b e r s (i.e., inverse axial con- d u c t a n c e ) , a n d Fig. 7 presents t h e results. As noted in Fig. 8, p o t a s - sium conductance [5] in axon cells also reaches limiting v a l u e s as
some essential system coefficient is changed experimentally from one value to a n o t h e r ; depolarization is t h e case in point. Again t h e q u a l i - t a t i v e similarity between experiment a n d c o m p u t a t i o n is noted. T h e fact t h a t one curve h a s a positive slope a n d t h e other a negative
Ο
2'·
6Ζ 1.5 Ο Η 1.4
<
ûc 1. 3
<
"Ό
0 . 0 5 0.10 0.15 0 . 2 0 0 . 2 5 0 . 3 0 Ι / ΝFIG. 7. Long-time limiting values of separation factor versus axial diffusivities computation by numerical integration of Eqs. (1-5) (axial diffusivities φ and η are proportional to the variable 1/JV).
one is n o t r e l e v a n t here, b u t t h e fact t h a t both systems t e n d to reach finite limiting conditions is suggested to be relevant.
T o t a l ionic nerve cell currents, [4] after a change in bias voltage show a m i n i m u m in t h e t r a n s i e n t curve. Figure 9 shows t h a t c o m p u t a -
D E P O L A R I Z A T I O N , ( M.V. )
FIG. 8. Long-time limiting values of potassium conductance versus depolari- zation from Hodgkin and Huxley [5] on squid axon cells.
tional integrations also can lead to curves with m i n i m a (or m a x i m a ) with p a r a m e t r i c pumping. T h e curve in question is t h e m e a n s e p a r a - tion t r a n s i e n t as t h e system b o u n d a r y conditions in Of are changed in a step fashion. After initial conditions h a v e been wiped out, here
also a succession of superimposable curves are obtained only one of which is presented. I n t h e case of t h e r m a l p a r a m e t r i c pumping, the curve m i n i m a or m a x i m a arise because of r a p i d changes in local ad- sorptive equilibria which occur before longer-time axial column com- positional r e a r r a n g e m e n t s can t a k e p l a c e ; both actions occur in r e - sponse t o newly imposed t e m p e r a t u r e b o u n d a r y conditions.
I n m a k i n g t h e assumption t h a t a p a r a m e t r i c a c t i v e - t r a n s p o r t p u m p is associated with the experimental responses of axon cells as given in Fig. 5 , one is confronted with a problem of characteristic time constants. T h e time constant in question is of t h e order of a
Q: ο
Ι - Ο
<
1.4
<
LU c/)
1.0
\ ν -V, AS YMP1 ΌΤΕ_
0 5 10 15 2 0 2 5
NO. OF CYCLES AFTER CHANGE
FIG. 9. Mean separation factor versus time (number of cycles after change) computed by integration of Eqs. (1-5) for step function alternations in the value of the system boundary condition in ef (response to only one-step change is depicted). Total ionic nerve cell currents have been observed [4] to show minimum in transient curve after change in bias voltage.
millisecond; by contrast, time constants in t h e work of Caldwell et al. [1] and of H o d g k i n and K e y n e s [7] on direct ion diffusion m e a - surements when metabolism is disturbed b y the presence of t e m p o r a r y or p e r m a n e n t poisons is of t h e order of 10
3
seconds. T h e a p p a r e n t discrepancy m a y p e r h a p s be rationalized by a conceptual model exten- sion within t h e framework of p a r a m e t r i c pumping. Consider each end of t h e p u m p system (i.e., m e m b r a n e or similar structure) to be sur- mounted by a receiver ( i n t r a - or extracellular space) which receives solute substance only by the processes of diffusion. I n effect the p u m p locally becomes an open system with a F i c k i a n receiver a t its two boundaries. I n such an assembly there can be a quick initial response, such as in Figs. 5 and 6 in which a r a p i d b o u n d a r y layer g r a d i e n t
is established, a high, (almost) limiting-value solute concentration being built up at the boundary between pump and diffusion systems.
This high, locally pumped-up solute concentration can then serve as a source for "filling u p " by diffusion the relatively large total volume of the biological organ, such as an axon cell, this last process having a relatively long over-all time constant.
Order of magnitude figures m a y be of interest in connection with the time constants under discussion. L
2
/2t = D, the Einstein equation characteristic of diffusion, is employed for this purpose. (L is the mean diffusion distance, t is the duration time of diffusion, and D is the diffusion constant.) T a k e the squid axon radius (0.05 cm) to be the diffusion p a t h length characteristic of the organ and let the response time be 10
3
seconds. A computed diffusion constant thus is about 10"
6 cm
2
/sec. Diffusion constants in water ordinarily range between 10~
4
and 10~
5 cm
2
/sec. Presumably diffusion in colloidal axo- plasm is smaller than in water and to this extent the estimate seems to be self consistent. Unfortunately there is little work on direct diffu- sion measurements in cell fluids with which to make comparison. T u r n - ing now to short-time penetration and accepting for the moment the figure for D of 1 0
-6 cm
2
/sec, one m a y calculate the mean diffusion distance in 1 millisecond to be about 10"
4
· 5
cm (5000 Â ) .
Possible Model Discrimination through Experiment If some form of parametric pumping is involved in active trans- port, then all dependent variables would be expected to oscillate about a mean, which itself can be a transient or be stationary in the limit of large elapsed times, as illustrated in Fig. 2. Thus, in experiments of the type that underlie the data of Fig. 3, one should seek to deter- mine by direct measurement whether oscillations are superimposed on mean values. Electrical outputs are particularly convenient in this connection because, with present instrumentation, frequencies as high as 5 Χ 1 0
10
cps m a y be detected. If in such m e a s u r e m e n t s oscillations were found to persist over a long period of time, coordinated behavior in t h e entire s t r u c t u r a l u n i t might well be presumed. On t h e other hand, if pulsations were to decay after being initiated by step changes in voltage clamp experiments, t h e presence of synchronous behavior, which subsequently becomes randomized, might be hypothesized.
A second t y p e of experiment is related to chemical p e r t u r b a t i o n s . Effects of adding metabolic inhibitors, of adenosine t r i p h o s p h a t e
( A T P ) , and other substances, h a v e been studied in relation to t h e
energy source for active diffusion, a n d generally experiments h a v e involved direct m e a s u r e m e n t of ion transfer r a t e s . Such additives m i g h t well be applied in s t u d y i n g r a p i d - a c t i n g electrical o u t p u t s , such as are illustrated in Fig. 5, both with regard to influence on t h e t r a n - sient response of m e a n p o t e n t i a l values a n d on t h e superimposed oscil- lations, if a n y . F u r t h e r m o r e , if w a t e r transfer t h r o u g h m e m b r a n e s should be involved, t h e s t u d y of hormones a n d diuretics on t h e electri- cal o u t p u t s might be instructive.
C O N C L U S I O N S
1. P a r a m e t r i c p u m p i n g , a n oscillation-driven separation process, is described. E x p e r i m e n t a l separations have been achieved for N a C l - w a t e r and a m a t h e m a t i c a l model has been written and solved numerically. P a r a m e t r i c p u m p i n g is suggested t o be a form of active diffusion; as presently performed a spatial m e a n concentration difference is caused to develop in a column of a d s o r b e n t particles a t t h e expense of t h e r m a l energy.
2. P a r a m e t r i c p u m p i n g concepts are generalized a n d extended as a possible model for active t r a n s p o r t in biological cells.
3. Responses of numerical solutions of a minimum-essential set of p a r a m e t r i c p u m p i n g equations are compared with classic squid axon, voltage clamp, ion t r a n s p o r t experiments. I t is con- cluded t h a t curve shapes, s o m e w h a t similar to those generated experimentally, can be developed by t h e m a t h e m a t i c a l model in question.
4. E x p e r i m e n t s are suggested which m i g h t serve as a basis for verification, rejection or modification of p a r a m e t r i c p u m p i n g
as a c a n d i d a t e model for active biological t r a n s p o r t .
ACKNOWLEDGMENTS
I thank Alan Rice for valuable discussions and for assistance in numerical analyses.
The work is supported through Grant GP-2286 of the National Science F o u n - dation, Engineering Division. An initiation grant from the Research Board of Princeton University is acknowledged, as is also the use of a digital computer in the Princeton University Computer Center which is supported in part by National Science Foundation Grant NSF-GP-579.
REFERENCES
1. Caldwell, P . C , Hodgkin, A. L., Keynes, R. D., and Shaw, T. I., Physiol.
(London) 152, 561 (1960).
2. Eisenman, J., in " M e m b r a n e Transport and Metabolism" (A. Kleinzeller and A. K o t y k , eds.), p. 163. Academic Press, New York, 1960.
3. Helfferich, F., "Ion Exchange." McGraw-Hill, New York, 1962.
4. Hodgkin, A. L., Science 145, 1148 (1964).
5. Hodgkin, A. L., and Huxley, A. F., J. Physiol. {London) 117, 500 (1952).
6. Hodgkin, A. L., and Katz, B., / . Physiol. (London) 108, 37 (1949).
7. Hodgkin, A. L., and Keynes, R. D., J. Physiol. (London) 128, 28 (1955).
8. Huxley, A. F., Science 145, 1154 (1964).
9. Kurella, G. Α., in " M e m b r a n e Transport and Metabolism" (A. Kleinzeller and A. Kotyk, eds.), p. 54. Academic Press, New York, 1960.
10. Lousell, W. H., "Coupled Mode and Parametric Electronics." Wiley, New York, 1960.
11. Teorell, T., J. G en. Physiol. 42, 847 (1959).
12. Wilhelm, R. H., Rice, A. W., and Bendelius, A. R., Ind. Eng. Chem. Funda- mentals 5, 141 (1966).


![FIG. 5. Voltage clamp experiments on squid axon cells by Hodgkin and Huxley [5] expressed as potassium conductance versus time](https://thumb-eu.123doks.com/thumbv2/9dokorg/1138431.81154/16.648.133.522.375.551/voltage-experiments-hodgkin-huxley-expressed-potassium-conductance-versus.webp)

![FIG. 8. Long-time limiting values of potassium conductance versus depolari- depolari-zation from Hodgkin and Huxley [5] on squid axon cells](https://thumb-eu.123doks.com/thumbv2/9dokorg/1138431.81154/18.648.223.427.566.734/limiting-values-potassium-conductance-depolari-depolari-hodgkin-huxley.webp)