PROSTAGLANDINS
John L. Humes
GENEPAL INTRODUCTION
Mononuclear phagocytic cells synthesize and release a series of arachidonic acid (AA) oxygénation products. We have reported that resident mouse peritoneal macrophages, when exposed to inflammatory stimuli in cell culture, synthe- size and release prostaglandins (PG), principally PGE
2and 6-keto-PGFi
a, the stable metabolite of PGI2 (1, 2) . Other AA oxygénation products, including PGF2
a, thromboxanes, and hydroxy fatty acids, have also been reported to be produced by these cells (3, 4, 5) .
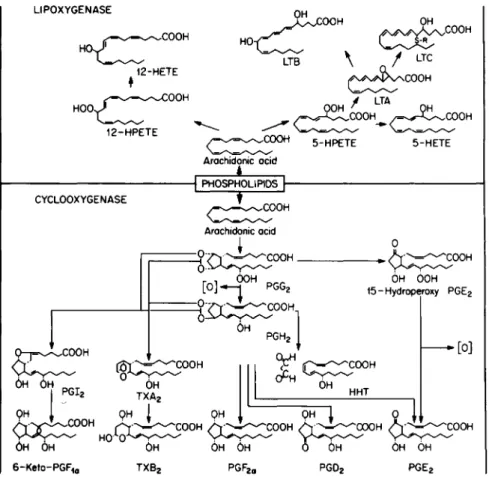
The deacylation of cellular phospholipids to liberate free AA initiates the synthesis of these various AA oxygénation products. The subsequent oxygénation of this fatty acid pro- ceeds by two independent pathways now termed the AA cascade
(Fig. 1). The cyclooxygenase branch of the cascade results in the formation of the stable primary PGs, i.e., PGs of the E, F, and D series. In addition, thromboxane Β~ (TXB^) and
METHODS FOR STUDYING Copyright © 1981 by Academic Press, Inc.
MONONUCLEAR PHAGOCYTES 6 4 1 All rights of reproduction in any form reserved.
ISBN 0-12-044220-5
LIPOXYGENASE OH
■COOH 12-HETE 12-HE
lOOJ1 k«^—N/
HO LTB
:OOH OH Ο ΛΛ
\ 7
LTC^\^S^sPv\C00H / LTA 12-HPETE
OOH ' OH /=v^rÂ^^COOH / = ^ - < ^ X O O H / ^ — ^ ν Χ Ο Ο Η 5-ΗΡΕΤΕ 5-HETE
CYCLOOXYGENASE
Arachidonic acid
k
PHOSPHOLIPIDS
f
COOHCOOH . COOH
[ 0 ] - ^ °H PGG:
OH OOH 15-Hydroperoxy PGE2
O-j=v^X00H
OH ÔH p r_ OH
P G I2 TXA:
QH ÔH OH
COOH
.COOH HO^O'
COOH, PGH2
f ^ ^ - ^ C O O H OH HHT
6-Keto-PGF1a
QH
"COOH /Y^^~"COOH
Ô H O H O H
TXB2 PGF2a
'[0]
COOH
Fig. 1. The arachidonic acid cascade.
6-keto-PGFla, the stable metabolites of labile TXA2 and PGI2, respectively, are formed through this pathway. All of these products arise from two labile endoperoxide intermediates, PGG2 and PGH2. Nonsteroidal antiinflammatory drugs, such as aspirin and indomethacin by interacting with the cyclooxygenase- peroxidase, inhibit the formation of these intermediates and thereby inhibit the synthesis of the primary PGs, 6-keto-PGF]_a, and TXB2. Also, an oxygen-centered radical [θ] is concomitantly produced in the peroxidatic conversions of PGG2 to PGH2 or
15-hydroperoxy-PGE2 to PGE2 (6).
In contrast, AA can also be oxygenated by indomethacin/
aspirin insensitive lipoxygenase pathways. One lipoxygenase branch of the AA cascade results in the synthesis of 12-L- hydroperoxy-5,8,10,14-eicosatetranoic acid (12-HPETE) and the
corresponding 12-hydroxy fatty acid (12-HETE). Recently, 12-HETE has been shown to be chemotactic for polymorphonuclear leukocytes (7). Alternatively, AA can be oxidized by another indomethacin/aspirin insensitive lipoxygenase to yield a series of 20-carbon fatty acids with one or two oxygen moieties and
containing three conjugated double bonds (8). The term leuko- triene has recently been proposed for these compounds (LTA, LTB, and LTC) (9). Recently, SRS-A has been shown to be leuko- triene C, with R being the thioester of reduced glutathione (10).
A discussion of the physiological, pathological, modulatory, and pharmacological effects of the stable oxygénation products of the AA cascade is beyond the scope of this article. However, the reader should appreciate that the stable products have been shown in many cases to be less active than their corresponding labile precursor(s). As an example, PGI2 is quite unstable with a 1/2 life of <5 min at 37°C and pH 7.4, yet this compound is extremely potent in preventing platelet aggregation. Its stable metabolite 6-keto-PGFia is without effect. Similarly, TXA2, with a 1/2 life of 0.5 min, is a potent inducer of platelet ag- gregation, whereas TXB2/ its stable metabolite, is devoid of this activity.
These various AA oxygénation products released by cells in culture can be measured in one of several ways: (1) bioassays;
(2) gas - liquid chromatography mass spectrometry; (3) release of radiolabeled products; and (4) radioimmunoassay. Only the bioassay methods allow an estimation of the labile, and thus perhaps most interesting, components. The principal advantage of bioassays is that they measure a definite physiological ac- tivity and thus can measure the labile intermediates formed in the AA cascade. It was only by the use of bioassay techniques that the discoveries of TXA2 and PGI2 were made (11, 1 2 ) . Most of these methods are based on the potent muscle contractile/
relaxant properties of these compounds. In addition, platelet aggregation has been used as a bioassay technique for products of AA metabolism. Many of these preparations are quite sensi- tive and require only submicrogram amounts of the test sub- stances. The specificity can be markedly improved by assaying the test substance on a combination of preparations, each having a different reaction to the known standard compounds. In addi- tion, various pharmacological receptor antagonists, notably an- tihistamines, a- and 3-adrenergic antagonists, and cholinergic antagonists, are added to further increase specificity. How- ever, the overwhelming disadvantage of these methods is their low sample capacity. The reader is directed to an excellent re- view of various bioassay techniques for AA metabolites (13).
The combination of gas - liquid chromatography coupled with mass spectrometry permits the most definite statement of com- pound structure. However, relatively large quantities of sample, i.e., >10 yg, are generally needed and the actual sample capaci-
ty is limited. Sample preparation is also time consuming since they must first be extracted, partially separated, and subse- quently derivatized. Also deuterium-labeled PGS, used as inter- nal standards, are not readily available and must be prepared.
This report will detail the radiorelease (the release of radiolabeled products from radiolabeled cells) and radioimmu- noassay methods as used in our laboratory. Both of these techniques are especially applicable for the detection and quantitation of PGs in cell culture. The former method allows the identification but not quantitation of a broad spectrum of radioactive AA metabolites. The latter method is complimentary to the former in that it allows precise quantitation of the in- dividual AA oxygénation products.
II. RADIORELEASE METHOD 3 14
[ H ] or [ c]AA is efficiently incorporated into cellular phospholipids of a variety of cells and tissue preparations.
Resident mouse peritoneal macrophages, the cells used in these studies, incorporate considerable amounts of
[3H]AAover 3 - 4 hr incubation periods in cell culture (2). Other cellu- lar preparations including platelets, lymphocytes, fibroblasts, as well as intact rabbit hearts and kidneys, have been shown to incorporate useful amounts of either [^c] or
[3H]AAinto cel- lular phospholipids for subsequent l^C-
o r3
H_i
aj
Dei
ecj p
roduct synthesis and release (14 - 17). The primary advantage of this method is that it measures de novo synthesis of an AA oxygéna- tion product and not the absolute amount of the product. In many cases this measurement of the change in de novo product synthesis is of greater magnitude than the corresponding meas- urement of the total amount of product. In addition, this method evaluates the synthesis from endogenous, and thus rele- vant, substrate pools.
Another advantage of the radiorelease method is its high
sample capacity. In our laboratory, we find that the radio-
release method has a greater sample through-put than correspond-
ing analysis by radioimmunoassay. In addition, the investiga-
tor can conveniently quantitate more than one AA oxygénation
product as the product derived from both the cyclooxygenäse or
lipoxygenäse enzymic pathways and can be separated one from the
other in a variety of Chromatographie systems. Clearly the
flexibility of being able to monitor conveniently more than one
product is an advantage not easily attained by the other assay
methods. As the identification of the released radiolabeled
product is assessed by comparing its mobility with authentic
standards, this method is not an absolute criteria of product
identification. The nature of the product should be further
verified by other independent assay methods, such as radioim-
munoassay. Positive structure identification must be proven by mass-spectral analysis when sufficient quantities of the product are available.
A. Reagents
Male SW/ICR specific pathogen-free mice are purchased from Hilltop Lab Animals, Inc., Scotdale, Pennsylvania, and main- tained on a standard pellet diet and water ad libitum.
The cell culture reagents are purchased from Grand Island Biological Co., Grand Island, New York. The porcine serum is inactivated by heating at 56°C for 30 min (HIPS). Zymosan is from ICN/K and K Labs, Plainfield, New York. The zymosan par- ticles are suspended in phosphate-buffered saline (PBS), boiled, and centrifuged 3 times. The final pellet is suspended in PBS at a concentration of 20 mg/ml and stored frozen. The stock so- lution is diluted in culture medium (usually 1:10) and sonicated immediately before addition to the cell cultures. Nunclon cul- ture dishes, 60 mm, are from Vangard International, Inc., Nep- tune, New Jersey. Gentamicin is from the Schering Corp., Kenilworth, New Jersey.
[5,6,8,9,11,12,14,15- H]arachidonic acid, specific activity 62 Ci/mmol, is obtained from New England Nuclear Corporation, Boston, Massachusetts. The hexane solution, 100 yCi/ml, is stored under N2 at -20°C and is stable for at least four weeks.
Reagent grade solvents from J. T. Baker, Phillipsburg, New Jersey are used for extractions and chromatography. The chromatography is routinely performed on Whatman SG-81 silicic acid impregnated Chromatographie paper from Ace Scientific, Linden, New Jersey. The sheets, 46 x 57 cm, are cut into
2 x 28 cm strips. PGE2/ PGF2
a(Ono Pharmaceuticals, Osaka, Japan), and arachidonic acid (Sigma, St. Louis, Missouri) are used as internal Chromatographie standards. A methanol solution of these standards containing 3 mg PGE2/ 3 mg AA, and 5 mg PGE2 per ml is prepared and stored at -20°C.
B. Procedures 1. Cell Culture
Resident mouse peritoneal macrophages are collected by peri-
toneal lavage from untreated pathogen-free mice and cultured as
previously described (2). Briefly, the lavaging medium is M 199
containing 1% heat-inactivated porcine serum (HIPS), 100 U of
penicillin and streptomycin/ml and 20 U heparin/ml. The cells
are plated at approximately 5 x 10
6cells/50 mm culture dish and
incubated for 2 hr at 37°C in 5% C0
oin air. The nonadherent
cells are removed by washing the adherent cell sheets with five 5-ml volumes of phosphate-buffered saline (PBS) using a mech- anized cell washing apparatus designed by P. D. Wightman of this laboratory. The adherent cells are maintained overnight in 4-ml M 199 containing 10% HIPS and 100 yg gentamicin/ml.
2. Prelabeling Macrophage Phospholipids with [^Η]ΆΆ After the overnight incubation, the cells are washed with PBS as described above and then incubated for 4 hr with 1 yCi
[3H]AA in 4 ml M 199 containing 1% HIPS. This medium is pre- pared by evaporating the [3HJAA hexane stock solution to dry- ness under nitrogen and dissolving the material in DMSO at 1000 times the desired concentration. The DMSO solution is subse- quently diluted 1:1000 in M 199 containing 1% HIPS to yield the desired concentration of 1 yCi/4 ml. This amount of DMSO, 0.1% has no effect in this system. Under these conditions the incorporated [3H ] A A is primarily found in cellular phospholipids
(phosphatidylcholine and phosphatidylethanolamine) and tri- glycérides (2) .
3. [ H\PG Release
After 4 hr the adherent cells are washed as previously des- cribed to remove unincorporated [3H]AA. T W O ml of M 199 devoid of serum but containing the various stimuli, such as zymosan, is added. Under these conditions, the release of [3H]PGE2 and
[^H]6-keto-PGF^a is linear for 6 - 8 hr. At the termination of the experiment, usually 4 hr, the media are collected.
4. Extraction and Chromatography of [3H]AA Oxidation Products
The culture media are acidified to 0.03 M with citric acid and PGE2 (50 yg), PGF2 a (30 y g ) , and AA (30 yg) are added
(10 yl of the methanolic stock solution). The acidified media are then extracted with three successive 3-ml aliquots of chloroform/methanol (2:1, v:v). The combined organic phases are washed with two successive 2-ml aliquots of methanol water
(2:1, v:v) and evaporated to dryness under a stream of N2 at 45°C. The residue is dissolved in 50 yl of ethylacetate/
methanol (3:1, v:v) and quantitatively applied to 2 x 28 cm strips of SG-81 paper. The strips are developed by descending chromatography with ethyl acetate/acetic acid (99:1, v:v). In some experiments, after the chromatograms are developed in this system, the strips are redeveloped in the same direction with 73-hexane/diethyl ether/acetic acid (75:25:1 by vol.). The in- ternal PG and AA standards are detected by exposure to I2 vapor.
In some experiments the radioactivity profile of the entire chromatogram is determined by scanning with a Packard 7201 radiochromatogram scanner. An alternative is to cut the chromatogram with scissors into 0.5-cm segments. The radioac- tivity of each individual segment is determined by counting the segment in 10-ml Aquasol in a Packard 3225 liquid scintillation spectrometer. In most experiments the area corresponding to PGE2 standard is cut out and counted. In a similar manner, other areas of the chromatogram, defined by their relative mo- bility to the added internal standards (PGE2, PGF2 a, AA) are cut out and counted.
D. Radioscan of [ H]Arachidonic Acid Oxygénation Products
Resident mouse peritoneal macrophages are labeled with [3H ] A A for 4 hr. As shown in Fig. 2b, the addition of 50 yg zymosan/ml for 4 hr caused the release of a variety of H- labeled components. The major 3H-components synthesized were[3H]PGE2 and [3H]6-keto-PGFla (6KF). Smaller amounts of labeled components coChromatographie with HETE, as well as other unidentified components more polar than this hydroxy fatty acid are also formed. Indomethacin, 10 yg/ml, completely inhibited [3H [PGE2 and [3H]6-keto-PGFla synthesis (Fig. 2 c ) . In the absence of zymosan no defined [3H]AA oxygénation products are released from the cells (Fig. 2 a ) .
Ill. RADIOIMMUNOASSAY
The first application of radioimmunoassay (RIA) techniques for measuring PGs was introduced by Levine and VanVunakis in 1970 (18). Since that time the use of this method has in- creased and a large literature now exists on the use of RIA to measure PGs and PG metabolites from a variety of cells, tis- sues, and body fluids. The reader is directed to the excellent review by Granström and Kindahl describing the use of RIA for measuring PGs and other AA oxygénation products (19).
RIA are exquisitively sensitive having limits of detection as low as 100 pg. In addition, these techniques have a large sample capacity. However, the poor specificity of RIA as ap- plied to PG measurements continue to be a major drawback. Al- though the specificity of the antibody is usually very high with respect to the cross-reactivity with known PGs, the data obtained with RIA techniques suggest that unknown substances in the samples may interfere. In many cases the absolute values obtained by RIA techniques have been considerably greater than
o o
■6
£
Ha)
W
1 No Addition
1
IW^MMM*
J 6KF PGF—PGE TXB-PGD
wv
· ·
H ETE AA '
o o '■5 σ ce
[(bl·
M (
1 Zymosan
) \J\AJI 1 *W
6KF PGF 2 PGETXB
V 3 f
PGD
\hf
4 5VHH
HETE AA
cc o
( c ) (I Zymosan + Indomethacin
6KF PGF PGE TXB PGD HETE AA
Fig. 2. Radioscan of released arachidonic acid oxygénation products. The organic extracts of the culture media are
chromatographed on SG-81 paper with ethyl acetate/acetic acid (99:1, v:v). The papers are dried and then rechromatographed in the same direction with n-hexane/diethyl-ether/acetic acid
(75:25:1 by vol.). The entire chromatogram is scanned with a Packard 7201 radiochromatogram scanner. Reproduced from Bonney et al. (2) with permission.
corresponding values obtained by gas - liquid chromatography/
mass spectrometry.
To achieve a high degree of reproducibility, it is obliga- tory to use exclusively disposable labware and Nanograde or spectrograde solvents for extractions. To achieve utmost ac- curacy, the samples should be purified on silicic acid volumes or thin-layer chromâtography prior to the RIA. Every conceiv- able control incubation (i.e., medium alone and test substances, etc.) should be analyzed. By appreciating these specificity pitfalls, due to the high sensitivity of the RIA technique, one can obtain useful and meaningful PG mass analysis.
Many radioactive ligands are commercially available. Tri- tiated 'PGE
1, PGE
2, PGF
la, PGF
2a, TXB
2, and 6-keto-PGF
lawith high specific activity are available from New England Nuclear, Boston, Massachusetts. Antisera and in some cases complete kits for analysis of primary PGs can be purchased from various suppliers.
A. Reagents
Nanograde methanol, acetone, and ethyl acetate are from Mallinkrodt, St. Louis, Missouri. Silica gel thin layer chromatography plates 250 ym are from Analtech, Newark, Dela- ware. The plates are prewashed with ethyl acetate immediately prior to use. Antiserum to PGE
2, lot 61-335, is from Miles Laboratories, Elkart, Indiana. The lyophilized preparation is reconstituted with 8 ml PET buffer containing 0.1% gelatin.
The PET buffer is 10 mM potassium phosphate, pH 7.3, containing 1 mM ethylenediaminetetraacetic acid disodium salt and 0.25 mM thimerasol.
[5,6-
3H]PGE
2, specific activity 80-100 Ci/mmol, was ob- tained from New England Nuclear Corp., Boston, Massachusetts.
A [
3H]PGE
2trace solution containing approximately 20,000 cpm/0.1 ml PET buffer containing 0.1% gelatin is prepared.
Unlabeled PGE
2standards are prepared in methanol so that 50 yl aliquots will contain 50 - 1000 pg.
Norit A charcoal is from Ace Scientific, Linden, New Jersey and Dextran T-70 was from Pharmacia, Sweden. The dextran- coated charcoal suspension is prepared as previously described
(20). Briefly, 3 gm Norit A charcoal is suspended in 100 ml 0.25% Dextran T-70 in PET buffer. The charcoal is allowed to settle out of solution and the supernatant fluid is removed by aspiration. This settling process is repeated two additional times and the charcoal finally suspended in 20 ml PET buffer.
This stock solution is subsequently diluted with PET buffer
(usually a 1:10 dilution) so that 1 ml of the diluted suspension
will remove >95% of the unbound [
3H]PGE
2without appreciably re-
moving the [
3H]PGE
2bound to the antibody.
B. Procedures 1. Cell Culture
Resident mouse peritoneal macrophages are isolated and maintained in cell culture as previously described except that the [ % ] A A is omitted. At the termination of the experiment the media are collected.
2. Extraction and Purification of PGs for RIA
The culture medium is acidified to 0.03 M with citric acid and ^5000 cpm [%]PGE2 is added as a recovery standard. The samples are extracted with three successive 3-ml aliquots of ethyl acetate. The combined organic phases were backwashed with two successive 1-ml aliquots of water and evaporated to
dryness under a stream of nitrogen at 40°C.
The sample residues are dissolved in 50 μΐ ethyl acetate/
methanol (3:1) and quantitatively applied to 1 cm lanes of a silica gel thin layer Chromatographie plate. On separate lanes, well separated from the lanes containing the samples, 10 yg each of PGE2 and PGF2a are spotted. The plate is developed 15 cm with ethyl acetate/acetone/acetic acid (90:10:1)'. The lanes
containing the samples are covered with glassine paper and the exposed lanes containing the PG standards are sprayed with a solution of 2% KMnC>4 in 1% Na2CC>3 to visualize the PG standards.
The silica gel from the area corresponding to the standard is removed and added to 0.5 ml water. The silica - gel - water mixture is extracted with two successive 2-ml aliquots of ethyl acetate and the combined ethyl acetate phase is evaporated to dryness under nitrogen at 45°C. The residues are dissolved in methanol for radioimmunoassay. An aliquot is counted for the recovery of the added [3H ] P G E 2 standard. The recovery through this procedure is routinely 50 - 70%.
3. Radioimmunoassays
The radioimmunoassays for PGE are performed by the method of Orczyk and Behrman (21). Briefly, aliquots of the methanolic sample solutions as well as aliquots of the methanolic PGE2
standards are evaporated to dryness under nitrogen. The samples and standards are incubated 30 min with 0.1 ml of the antisera solution at room temperature. The [3H ] P G E 2 trace solution, 0.1 ml, is then added and the mixtures incubated overnight at 4°C. The separation of antibody-bound [%]PGE2 from the un- bound [3H ] P G E2 is achieved by adding 1 ml of the diluted dextran-coated charcoal suspension. The charcoal suspension and assay tubes are maintained at 4°C for this operation. The assay tubes are mixed, incubated for 5 min at 4°C, and then
centrifuged at 4°C at 4000 g for 15 min. The supernatant phases, containing the antibody-bound-PGE, are decanted into 9-ml Aquasol and the radioactivity determined by liquid scin- tillation spectrophotometry. The resultant data are analyzed by Scatchard analysis on a Wang 2200 computer.
IV. CONCLUDING REMARKS
Cultures containing 5 x 1 0b resident peritoneal macrophages are prelabeled with [%]AA and subsequently challenged for 4 hr with zymosan as described in Section II. Sister cultures are maintained in an identical fashion except that [3H ] A A was
omitted. The medium is collected and analyzed for PGE2 by both radiorelease and RIA techniques. As shown in Table I, the zy- mosan induced increase in PGE2 synthesis and release was ap- proximately equal as evaluated by both methods.
Macrophages maintained in cell culture constitute a useful model in which to study the AA cascade at the cellular level.
Resident mouse peritoneal macrophages synthesize and release large amounts of PGE2 when exposed in culture to various in- flammatory stimuli such as zymosan, phorbol myristate acetate, and antigen-antibody complexes (1, 22, 23). The synthesis and release of PGE2/ as well as the other stable cyclooxygenase products elicited by these agents, is inhibited by both non- steroidal and steroidal antiinflammatory agents. The non-
steroidal drugs inhibit the cyclooxygenation of AA, whereas the steroidal agents inhibit the deacylation of AA from phospholipid stores.
It has been clearly demonstrated that PGs of the E series modulate both lymphocyte function and proliferation (24, 25).
Consistent with these observations, inhibitors of cyclooxygenase have been shown to stimulate mitogen-driven thymidine incorpora- tion in unfractionated spleen cell suspensions. Thus it would appear that cyclooxygenase-derived products from adherent macro- phages may act alone or in concert with other soluble mediators to modulate and control immune function.
In addition to the products derived from the cyclooxygenase pathways, products derived from the lipoxygenase pathways are becoming of increasing biological interest. 12-HETE has been shown to be chemotactic for human leukocytes and is produced by mouse peritoneal macrophages (5, 7 ) . It is reasonable to specu- late that other lipoxygenase products with interesting biologic activities will be found to be produced by mononuclear phago- cytic cells.
In our laboratory we find the radiorelease method to be especially useful as it has large sample capacity and evaluates
TABLE I. Zymosan Stimulates PGE Synthesis and Release from Resident Mouse Peritoneal Macrophages as Evaluated by Both Radiorelease and RIA Methods9-
Radiorelease -Fold -Fold (cpm yg DNA) increase (ng/\ig DNA) Increase
No additions 18 ± 2 1 0.19 ± 0.09 1 Zymosan 50 ]ig/ml 349 ± 22 19 4.8 ±0.7 25 Zymosan 300 \ig/ml 1053 ±56 58 9.5 ± 1.1 50
aThe DNA content was 17 \ig/culture. The incubation was for 4 hr.
de novo product synthesis. The method is particularly suitable for pharmacological studies concerning the regulation of the AA cascade. However, this method does not yield absolute product amounts or the definite proof of structure. Thus to further characterize the PGE2 released by resident mouse peritoneal mac- rophages, we employ RIA to quantify the mass of PGE as well as gas - liquid chromatography mass spectrometry to prove the structure of this component. Only by such a combination of various analytical techniques will the regulation, identity, and
function(s) of the various products of the AA cascade be fully appreciated.
REFERENCES
1. J. L. Humes, R. J. Bonney, L. Pelus, M. E. Dahlgren,
S. Sadowski, F. A. Kuehl, Jr., and P. Davies. Macrophages synthesize and release prostagiandins in response to in- flammatory stimuli. Nature 269: 149-151, 1977.
2. R. J. Bonney, P. D. Wightman, P. Davies, S. J. Sadowski, F. A. Kuehl, Jr., and J. L. Humes. Regulation of prosta- glandin synthesis and of the selective release of lysoso- mal hydrolases by mouse peritoneal macrophages. Biochem.
J. 176: 433-442, 1978.
3. D. Gordon, M. A. Bray, and J. Morley. Control of lympho- kine secretion by prostaglandins. Nature 262: 401-402, 1976.
4. K. Brune, M. Colatt, H. Kalin, and B. A. Peskar. Pharma- cological control of prostaglandin and thromboxane release from macrophages. Nature 274: 261-263, 1978.
5. M. Rigaud, J. Durand, and J. C. Breton. Transformation of
arachidonic acid into 12-hydroxy-5,8,10,14-eicosatetraenoic acid by mouse peritoneal macrophages. Biochim. Biophys.
Acta 573: 408-412, 1979.
6. R. W. Egan, J. Paxton, and F. A. Kuehl, Jr. Mechanism for irreversible self-deactivation of prostaglandin synthetase.
J. Biol. Chem. 251: 7329-7335, 1976.
7. E. Goetzl and R. R. Gorman. Chemotactic and chemokinetic stimulation of human eosinophil and neutrophil polymorpho- nuclear leukocytes by 12-L-5-8-10-heptadecatrienoic acid
(HHT). J. Immunol. 120: 526-531, 1978.
8. P. Borgeat and B. Samuelsson. Metabolism of arachidonic acid in polymorphonuclear leukocytes. J. Biol. Chem. 254:
7865-7869, 1979.
9. B. Samuelsson, P. Borgeat, S. Hammarström, and R. C. Mur- phy. Introduction of a nomenclature: Leukotrienes.
Prostaglandins 17: 785-787, 1979.
10. S. Hammarström, R. C. Murphy, B. Samuelsson, D. A. Clark, C. Mioskowski, and E. J. Corey. Structure of leukotriene C identification of the amino acid part. Biochem. Biophys.
Res. Commm 91: 1266-1272, 1979.
11. M. Hamberg, J. Svensson, and B. Samuelsson. Thromboxanes:
A new group of biologically active compounds derived from prostaglandin endoperoxides. Proc. Nat. Acad. Sei. USA
72: 2994-2998, 1975.
12. S. Moncada, R. Gryglewski, S. Bunting, and J. R. Vane.
An enzyme isolated from arteries transforms prostaglandin endoperoxide to an unstable substance that inhibits plate- let aggregation. Nature 263: 663-665, 1976.
13. S. Moncada, S. R. Ferreira, and J. R. Vane. Bioassay of prostaglandins and biologically active substances derived
from arachidonic acid. In "Advances in Prostaglandin and Thromboxane Research," Vol. 5 (J. C. FrÖlich, ed.), pp.
211-236. Raven Press, New York, 1978.
14. T. K. Bills, J. B. Smith, and M. J. Silver. Metabolism of [ c] arachidonic acid by human platelets. Biochim.
Biophys. Acta 424: 303-314, 1976.
15. C. W. Parker, W. F. Stenson, M. G. Huber, and J. P. Kelly.
Formation of thromboxane B2 and hydroxyarachidonic acids in purified human lymphocytes in the presence and absence of PHA. J. Immunol. 122: 1572-1577, 1979.
16. S.-C.L. Hong and L. Levine. Stimulation of prostaglandin biosynthesis by bradykinin and thrombin and their mechanism of action on MC5-5 fibroblasts. J. Biol. Chem. 251: 5814-
5816, 1976.
17. P. C. Isakson. A. Raz, and P. Needleman. Selective incor- poration of [ c]arachidonic acid into the phospholipids of intact tissues and subsequent metabolism to [-^c]prosta- glandins. Prostaglandins 12: 739-749, 1976.
18. L. Levine and H. Van Vunakis. Antigenic activity of pros-
taglandins. Biochem. Biophys. Res. Commun. 41: 1171-1177, 1970.
19. E. Granström and H. Kindahl. Radioimmunoassay of prosta- glandins and thromboxanes. In "Advances in Prostaglandin and Thromboxane Research," Vol. 5 (J. C. Frölich, ed.), pp. 119-210. Raven Press, New York, 1978.
20. H. R. Behrman, K. Yoshinaga, H. Wyman, and R. O. Greep.
Effects of prostaglandins on ovarian steroid secretion and biosynthesis during pregnancy. Am. J. Physiol. 221:
189-194, 1971.
21. G. P. Orczyk and H. R. Behrman. Ovulation blockade by aspirin or indomethacin - in vivo evidence for a role of
prostaglandins in gonadotrophin secretion. Prostaglandins 1: 3-20, 1972.
22. J. L. Humes, P. Davies, R. J. Bonney, and F. A. Kuehl, Jr.
Phorbol myristate acetate (PMA) stimulates the release of arachidonic acid and its cyclooxygenase products by macro- phages. Fed. Proc. 37: 1318, 1978.
23. R. J. Bonney, P. Naruns, P. Davies, and J. L. Humes.
Antigen-antibody complexes stimulate the synthesis of re- lease of prostaglandins by mouse peritoneal macrophages.
Prostaglandins 18: 605-615, 1979.
24. H. R. Bourne, L. M. Lichtenstein, K. L. Melmon, C. S.
Henney, Y. Weinstein, and G. M. Shearer. Modulation of inflammation and immunity by cyclic AMP. Science 184:
19-28, 1974.
25. J. S. Goodwin, R. P. Messner, and G. T. Peake. Prosta- glandin suppression of mitogen-stimulated lymphocytes in vitro. J. Clin. Invest. 62: 753, 1978.