https://doi.org/10.1007/s11120-021-00841-3 ORIGINAL ARTICLE
Singlet oxygen damages the function of Photosystem II in isolated thylakoids and in the green alga Chlorella sorokiniana
Faiza Bashir1,2 · Ateeq Ur Rehman1 · Milán Szabó1,3 · Imre Vass1
Received: 30 January 2021 / Accepted: 26 April 2021 / Published online: 19 May 2021
© The Author(s) 2021
Abstract
Singlet oxygen (1O2) is an important damaging agent, which is produced during illumination by the interaction of the triplet excited state pigment molecules with molecular oxygen. In cells of photosynthetic organisms 1O2 is formed primarily in chlorophyll containing complexes, and damages pigments, lipids, proteins and other cellular constituents in their environ- ment. A useful approach to study the physiological role of 1O2 is the utilization of external photosensitizers. In the present study, we employed a multiwell plate-based screening method in combination with chlorophyll fluorescence imaging to characterize the effect of externally produced 1O2 on the photosynthetic activity of isolated thylakoid membranes and intact Chlorella sorokiniana cells. The results show that the external 1O2 produced by the photosensitization reactions of Rose Bengal damages Photosystem II both in isolated thylakoid membranes and in intact cells in a concentration dependent man- ner indicating that 1O2 plays a significant role in photodamage of Photosystem II.
Keywords Singlet oxygen · Chlorophyll fluorescence imaging · Photoinhibition · Thylakoid membranes · Microalgae Abbreviations
Chl Chlorophyll PSI Photosystem I PSII Photosystem II RB Rose Bengal
Introduction
Photosynthesis is a basic process in the biosphere in which green plants, algae and cyanobacteria utilize solar energy to synthesize carbohydrates from carbon dioxide and water see (Renger and Govindjee 1985). The initial, light-depend- ent steps of the photosynthetic process are performed by four protein complexes embedded in the thylakoid mem- brane, namely Photosystem II (PSII), Photosystem I (PSI), cytochrome b/6 f complex and the ATP synthase. NADPH
and ATP, which are produced in the light-dependent reac- tions are utilized in the so-called dark reactions of photosyn- thesis, which leads to the fixation of atmospheric CO2 and result in the production of carbohydrates.
Although light is the ultimate driving force of photosyn- thesis, light at the same time is also a significant damaging agent, which can impair photosynthetic activity. This pro- cess is called photoinhibition, whose mechanism remains controversial despite intensive research during the last dec- ades (Jones and Kok 1966; Ohad et al. 1984; Barber and Andersson 1992; Aro et al. 1993; Nishiyama et al. 2006;
Vass and Cser 2009; Oguchi et al. 2009; Murata et al. 2012;
Tyystjärvi 2013; Zavafer et al. 2017, 2019). The most light- sensitive site in the photosynthetic apparatus is the PSII complex in which electron transport activity is impaired and the D1 (and D2) reaction centre protein is degraded, see (Aro et al. 1993). In addition to PSII, PSI (Sonoike and Terashima 1994; Sonoike et al. 1995; Suorsa et al. 2012;
Tiwari et al. 2016; Lima-Melo et al. 2019), as well as the Chl containing light harvesting antenna structures can also be damaged by light (Zolla and Rinalducci 2002; Rinalducci et al. 2004, 2008; Lingvay et al. 2020). The detrimental effects of light linearly depend on light intensity (Tyystjarvi and Aro 1996) and can occur at all light intensities (Keren et al. 1997; Kou et al. 2012), therefore plants have evolved a protective repair mechanism, by which light induced loss of
* Imre Vass
vass.imre@brc.mta.hu
1 Biological Research Centre, Institute of Plant Biology, Eötvös Loránd Research Network (ELKH), Szeged, Hungary
2 Doctoral School of Biology, University of Szeged, Szeged, Hungary
3 Climate Change Cluster, University of Technology Sydney, Sydney, Australia
photosynthetic activity can be restored. This repair mecha- nism proceeds via de novo synthesis of the light-damaged D1 (and D2) subunit(s) of PSII (Aro et al. 1993; Nixon et al.
2010; Komenda et al. 2012; Murata and Nishiyama 2018;
Li et al. 2018). Due to competing photodamage and repair processes net loss of PSII activity occurs when the rate of photodamage exceeds the rate of repair.
Although the exact mechanism of photodamage and its repair is not yet fully clarified there is a consensus in the literature that reactive oxygen species (ROS) are involved in the overall photoinhibition process (Barber and Andersson 1992; Anderson and Chow 2002; Nishiyama et al. 2006;
Krieger-Liszkay et al. 2008; Pospísil 2009, 2012; Vass 2011;
Fischer et al. 2013).
Singlet oxygen (1O2), which is one of the most impor- tant ROS, is an excited state of molecular oxygen which is highly reactive (Ogilby 2010). It damages proteins, lipids and nucleic acids, so it is an important reactive oxygen spe- cies (ROS) in biological systems. On the other hand 1O2 is also an important signaling molecule (Triantaphylidès and Havaux 2009). It is less stable than triplet oxygen (3O2), and may be formed in a number of ways; however, the typical way is by energy transfer from the triplet state of a photosen- sitized pigment or dye molecule (Hirakawa et al. 2011; Fis- cher et al. 2013). Production of 1O2 has been demonstrated in isolated photosynthetic complexes (Macpherson et al. 1993;
Hideg et al. 1994a, b; Telfer et al. 1999) and intact photosyn- thetic systems (Hideg et al. 1998; Flors et al. 2006; Rehman et al. 2013, 2016b). It has also been shown that photoler- ance in highly light tolerant algal species is accompanied by decreased 1O2 production (Treves et al. 2016; Virtanen et al. 2021). However, the exact sites and mechanisms of ROS action in photoinhibition are debated. According to one idea ROS, including 1O2, affects only the repair of PSII by inhibiting translation elongation of the psbA gene, thereby preventing de novo synthesis of photodamaged D1 protein in the cyanobacterium Synechocystis PCC 6803 (Nishiyama et al. 2001, 2004, 2006). Recent results however, have shown that 1O2 does not affect the PSII repair process in green algae (Dall’Osto et al. 2019; Barera et al. 2021). Moreover, it has also been proposed that β-carotene molecules, which are oxidized by 1O2 act as signaling agents that induce defense mechanisms that alleviate the effects of PSII photodamage conditions (D’Alessandro and Havaux 2019). Other studies have demonstrated a clear correlation between the rate of
1O2 formation and the rate of PSII photodamage in various experimental systems (Rehman et al. 2013; Hakkila et al.
2013, 2014; Bersanini et al. 2014; Sedoud et al. 2014; Treves et al. 2016) pointing to the role of 1O2 as an agent that dam- ages directly the function and structure of the PSII complex (Vass and Cser 2009).
One useful approach to assess to role of 1O2 in PSII photodamage is the generation of 1O2 by externally added
photosensitizers. A previous study has compared the ability of different 1O2 photosenzitizers (Rose Bengal, Methylene Violet, Neutral Red and Indigo Carmine) to penetrate into plant cells (Kovács et al. 2014). It was concluded that Rose Bengal (RB), which is localized in the chloroplasts and can be excited by green light with only minor effect on photo- synthetic electron transport, was the most efficient 1O2 pro- ducing photosensitizer that resulted in PSII activity loss and D1 protein degradation (Kovács et al. 2014). Similar results were obtained earlier also by using RB infiltrated tobacco leaves (Hideg et al. 2007). However, in these studies very high RB concentrations (100 μM, and 1 mM, respectively) had to be used to ensure infiltration into the tobacco leaf, and there was no information about the actual level of 1O2 inside the cells and in the thylakoid membranes. Therefore, the possibility that unnaturally high 1O2 levels caused unspecific PSII damage in these plant studies could not be excluded. It is of note that 1O2 production in the presence of low micro- molar RB concentrations has been shown to induce growth retardation and specific gene expression in the green alga Chlamydomonas reinhardtii (Leisinger et al. 2001; Fischer et al. 2004), however, direct PSII damage was not demon- strated in these studies.
In the present study we investigated the effect of 1O2, when produced by low concentrations of RB, on the photo- synthetic activity of isolated spinach thylakoids and intact Chlorella sorokiniana cells. Our data show a RB concentra- tion-dependent loss of PSII activity in both systems (in the 1–10 μM RB range), which confirm that externally generated
1O2 can directly inactivate the PSII complex.
Material and methods
Biological materialsChlorella sorokiniana cells (originating from Culture col- lection of Autotrophic Organisms (CCALA, Trebon, Czech Republic) were propagated in BG-11 growth medium (pH 7.5) and grown at 24 ℃ at the irradiance of 60–70 μmol photons m−2 s−1 white light, in 500 mL flasks containing 200 mL of BG-11 kept on an orbital shaker. Four days old cultures in the exponential growth phase (A750 of 0.2–0.3) were used for the experiments. The chlorophyll concen- tration (Chl a + b) was determined by a UV-1601 (SHI- MADZU) spectrophotometer after extracting the pigments with acetone:DMSO 1:1. Chl a + b content was calculated according to (Shoaf and Lium 1976). Cells were harvested by centrifugation at 6500 g for 5 min and re-suspended in 100 mL of fresh BG-11 medium at concentration of 5 μg of Chl mL−1. The cells were kept under normal growth condi- tions for one hour before measurement.
Thylakoid membrane preparation
Thylakoid membranes were isolated from fresh spin- ach leaves as described earlier by (Anderson 1981), and suspended in a buffer solution containing 50 mM tricine (pH = 7.5), 7 mM MgCl2, 7 mM CaCl2 and 0.3 M Sorbitol.
Thylakoid membranes were stored at − 80 °C for further experiments. Before measurements, thylakoid membranes were resuspended in the same buffer with the Chl concentra- tion of 5 μg of Chl mL−1.
Experimental procedure for the Rose Bengal assay The Rose Bengal (RB) stock solution (500 μM in distilled water) was freshly prepared before each experiment. Sam- ples (in triplicates) were incubated in the presence of 0, 1, 5 and 10 μM RB in 24 well plates (Vision Plate™ 24 Well, 4titude, Brooks Life Sciences, U.K.), which were placed in a temperature controlled incubation chamber (temperature was maintained at 24 ℃ for Chlorella cells and at 4 ℃ for iso- lated thylakoid membranes, using a water circulation heater- chiller, Julabo). The illumination was provided from the top by a LED array using green light (50 µmol photons m−2 s−1) in combination with white light (190 µmol photons m−2 s−1, green + white irradiance was 240 µmol photons m−2 s−1) to excite the RB dye in the 520–550 nm spectral range, without providing excess excitation to the photosynthetic processes.
In Chlorella cells the light treatment was performed both in the absence and presence of 300 μg mL−1 lincomycin, which inhibits protein synthesis dependent PSII repair. At the indi- cated time points, the well plates containing the samples were transferred for chlorophyll fluorescence imaging. The same experiment was also performed in complete darkness, under identical experimental conditions that were applied for light treatment but without applying the LED illumination.
Chlorophyll fluorescence imaging
and measurements of the maximum quantum yield of PSII
Chlorophyll fluorescence of the samples incubated in the well plates was assessed by pulse-amplitude modulated imaging (PAM) MAXI (Imaging-PAM M-series Chlorophyll Fluorom- eter Heinz Walz GmbH, Germany). The minimum fluores- cence in dark adapted state, F0, was measured using a weak measuring light (ML settings = 4, PPFD < 0.3 µmol photons m−2 s−1) and maximum fluorescence, Fm, was measured by applying a saturation pulse (SP setting = 10, length: 0.8 s, PPFD: approx. 2000 µmol photons m−2 s−1). The maximum quantum yield of PSII was calculated as Fv/Fm = (Fm − F0)/Fm. Samples were dark-adapted for 5 min before the measure- ments. The software ImagingWin was applied to select circular areas of interest for individual wells, in which F0 and Fm were
measured and Fv/Fm was calculated. The ML intensity was adjusted so that the F0 signal did not drop below 0.1, to allow reliable measurements of F0 and Fm. To ensure homogeneity of the chlorophyll fluorescence signal, an image correction was performed according to the manufacturer, which enabled homogeneous signal intensity across the imaged area. Despite all corrections, some heterogeneity of the incident irradiance in the individual wells always remains, therefore minor spatial variations in the amplitude of F0 and Fm cannot be completely avoided. However, this can be compensated by the ratio cal- culation of maximum quantum yield of PSII (Fv/Fm), which eliminates the spatial heterogeneity of the amplitude of the basic fluorescence parameters (Schreiber et al. 2007). RB in the thylakoid buffer or in the culture medium did not show any autofluorescence with the 450 nm ML and SP light source of the PAM system in the 1–10 μM concentration range, as RB does not absorb light in this spectral region, therefore, it does not interfere with the Chl fluorescence imaging measurements.
Oxygen evolution/uptake measurements
Oxygen evolution and uptake rate was measured using a 4-channel FireStingO2 (FSO2-4) fiber-optical oxygen meter with optode sensors (Robust oxygen probe, OXROB10) (PyroScience GmbH, Aachen, Germany). Oxygen measure- ments were performed in 1 × 1 cm plastic cuvettes, which were placed under identical illumination (green-white light, 240 µmol photons m−2 s−1) and incubation conditions that were applied for the multiwell plate experiments. The cuvettes were mounted 45° onto a plastic platform, to allow homogeneous light penetration to the samples. The optodes were placed into the cuvettes containing the samples and sealed with plastic stoppers. Before measurements, 2-point calibration was performed using air-saturated water and deoxygenated water (with Na2S2O4) according to the manu- facturers’ specifications. 2 mL cultures with the Chl content of 5 μg mL−1 were loaded into the cuvettes, and measure- ments in the presence or absence or RB, and in the presence or absence of lincomycin were performed as specified at the relevant sections (‘Results’ and ‘Discussion’). Dark respi- ration was measured for 5 min in complete darkness, then oxygen evolution/uptake rate (depending on the applied con- ditions) was measured for 5 min after switching on the light.
To determine 1O2 production by O2 uptake, 5 mM Histidine was applied. Oxygen evolution/uptake rates are expressed in μmol O2 mg Chl−1 h−1.
Statistical analysis
Statistical analysis was performed using OriginPro (OriginLab Corporation, Northampton, MA, USA). One- way analysis of variance (ANOVA) and Tukey’s post-hoc
multiple comparison tests (α = 0.05) were performed on independent samples to detect statistically significant differences between treatments. Normality tests were performed using Kolmogorov–Smirnov method and the homogeneity of variance test was performed using Lev- ene’s method.
Results
Externally produced 1O2 accelerates photodamage of PSII in isolated thylakoid membranes
To investigate the effect of externally generated 1O2 on pho- tosynthetic activity of isolated thylakoid membranes, sam- ples were illuminated in the presence of various concentra- tions of RB in 24-well plate and PSII activity was quantified by determining the Fv/Fm values in each well using vari- able Chl fluorescence imaging. This is a particularly useful approach when PSII activity has to be determined simultane- ously in a large number of samples (Schreiber et al. 2007).
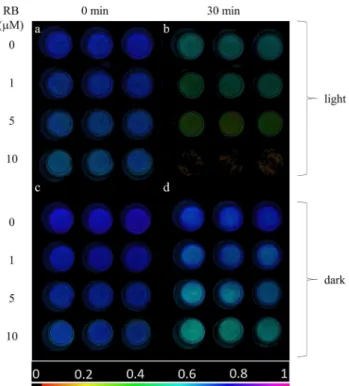
At the initial time point (t = 0), thylakoid membranes imaged in the well plates exhibited homogeneous Fv/Fm values of approx. 0.66–0.74, however, somewhat lower Fv/Fm was observed with increasing RB concentrations (Fig. 1a). Fv/Fm decreased during the light exposure in a RB concentration dependent manner; at 10 μM the Fv/Fm essentially dropped to 0 after 30 min (Fig. 1b). The Fv/Fm values also showed some decrease in darkness, which was more pronounced at higher RB concentrations (Fig. 1c, d). These findings dem- onstrate that illumination of isolated thylakoids in the pres- ence of RB induces the loss of PSII activity.
The detailed time course of Fv/Fm changes show that PSII activity was gradually decreased in the light-exposed thyla- koid membranes (Fig. 2a). Importantly, this effect showed a strong dependence on RB concentration. While in the absence of RB the thylakoids retained ca. 75% of their ini- tial activity even after 30 min of light exposure, in the pres- ence 10 µM RB a complete loss of Fv/Fm occurred (Fig. 2a).
The thylakoids which were treated with 1 and 5 μM RB showed an intermediate behavior. The Fv/Fm values showed some decrease even in darkness. This effect was negligi- ble in the absence of RB up to 30 min (95% of the initial activity remained), but was enhanced when RB was also present (80% remaining activity at 10 μM RB after 30 min) (Fig. 2b).
To compensate for the slow dark inactivation of PSII activity of thylakoids the Fv/Fm data are also shown after normalization of the data obtained in the light to those obtained in the dark (Fig. 2c). This representation shows the extent of PSII activity loss, which is actually caused by externally generated 1O2.
Histidine ameliorates the photodamaging effect of externally produced 1O2 in thylakoids
To check if the observed loss of PSII activity by illumination in the presence of RB is indeed related to 1O2-induced dam- age or not, His was also added to the thylakoid samples. His is a known chemical scavenger of 1O2 (Matheson and Lee 1979; Méndez-Hurtado et al. 2012), which can be used in photosynthetic systems without affecting electron transport (Rehman et al. 2013). The addition of 5 mM His together with 5 μM RB provided a significant protection against the loss of PSII activity when compared to the effect of RB addition alone (Fig. 3), confirming the involvement of 1O2 in PSII damage.
Externally produced 1O2 accelerates photodamage of PSII in intact Chlorella cells
To assess the effect of externally produced 1O2 in intact cells first we wanted to have an estimation of the 1O2 flux, which is produced by the illumination of RB. This was achieved by using the His-mediated chemical trapping method, which removes dissolved O2 from the medium as a results 1O2-induced oxidation of His, which results
Fig. 1 Fv/Fm image of thylakoid membranes illuminated with green + white light (panels a, b) or kept in darkness (c, d) for 0 min (a, c) and 30 min (b, d). In all panels first row represents control (0 µM RB), second row 1 µM RB, third row 5 µM RB, fourth row 10 µM RB (n = 3). Fv/Fm values are determined in the selected circu- lar areas of interest. The color bar indicates the relative intensity of Fv/Fm
in easily measurable O2 uptake (Rehman et al. 2013).
Using this approach we showed that the O2 evolution rate decreased in Chlorella cells in the presence of 5 mM His compared to control cells in which His was absent, showing a ca. 52.8 ± 4.2 μmol O2 mg Chl−1 h−1 rate of
1O2 production under the illumination conditions which were applied for the photoinhibition experiments. When Chlorella cells were illuminated in the presence of 1 μM RB the O2 evolution rate was significantly reduced due to chemical trapping of 1O2 by proteins and lipids in the cells. Addition of His together with RB resulted in a large O2 uptake (Fig. 4), which shows a ca. -760.8 ± 195.5 μmol O2 mg Chl−1 h−1 rate of 1O2 production in the presence of RB. This 1O2 production rate in the bulk medium is
significantly higher than the 1O2 rate, which arises from the photosynthetic pigments, i.e. in the absence of RB.
However, although RB has been shown to reach chloro- plasts in intact leaves (Kovács et al. 2014), its local con- centration is most likely lower in the chloroplasts of the Chlorella cells than in the bulk medium. Therefore, the
1O2 concentration in the vicinity of PSII is expected to be somewhere between the ambient level, produced by the photosynthetic pigments in the absence of RB, and the bulk level in the culture medium.
Fig. 2 Effect of RB on the maximum quantum efficiency of PSII (Fv/Fm) of thylakoid membranes treated with green-white light (a), or kept in dark (b) for the indicated time periods. Panel c represents the ratio of Fv/Fm recorded during the light treatment or dark incuba-
tion experiment [Fv/Fm values in panel (a) were divided by the values on panel (b)]. Thylakoid membranes were incubated in 0 µM (black), 1 µM (red), 5 µM (blue) or 10 µM RB (green)
Fig. 3 Effect of histidine on RB-induced loss of PSII activity. Thy- lakoids were illuminated for 60 min in the absence and presence of 5 μM RB as in Fig. 1, either without further addition, or in the pres- ence 10 mM histidine. The Fv/Fm values are shown after correction for the change that occurs during dark storage of samples. Different letters above the bars indicate significant differences (p < 0.05)
Fig. 4 Oxygen evolution/uptake of Chlorella cells. Black, no treat- ment, blue, 1 μM RB treatment. Closed bars, no His, checked bars, in the presence of 5 mM His
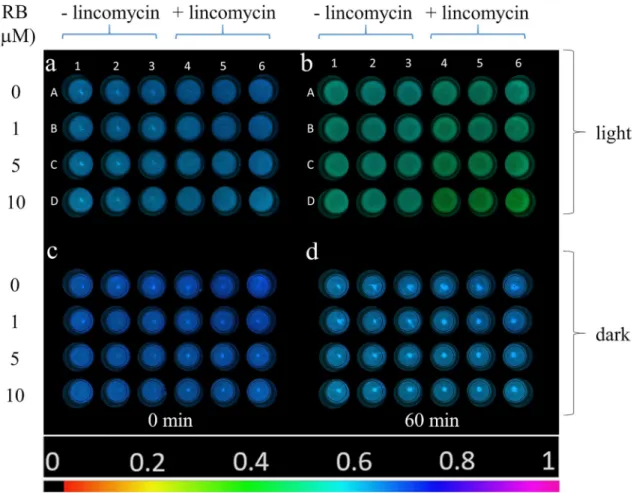
To clarify the effect of externally generated 1O2 on PSII activity in vivo, intact Chlorella cells were incubated in light and in darkness in the presence of different concentrations of RB. In intact systems photodamage and repair of PSII pro- ceeds in parallel. To separate these two processes, the exper- iments were performed both in the absence and presence of lincomycin, which blocks protein synthesis dependent PSII repair and thus allows the investigation of PSII photodamage without the effect of the ongoing repair. At the beginning of the experiment (t = 0 min) the cells exhibited similar Fv/Fm values (0.63–0.65) in the entire plate, indicating that the RB treatment did not affect maximal PSII quantum yield (Fig. 5a). After 60 min light treatment, a RB-concentration dependent decrease in Fv/Fm could be observed, which was more pronounced in the presence of lincomycin (Fig. 5b, well columns 4–6). When the cells were incubated in com- plete darkness only a minor decrease in Fv/Fm was observed, which was independent of RB concentration (Fig. 5d).
The detailed time course of Fv/Fm values shows that in the absence of lincomycin PSII activity loss showed a tendency to increase after 60 min light exposure in the presence of RB
(Fig. 6a). However, this effect was statistically significant only after 60 min light exposure (see below, Fig. 7). The pres- ence of lincomycin accelerated PSII damage, which became clearly visible already after 30 min (Fig. 6a). The enhance- ment of PSII photodamage showed a clear dependence on RB concentration. While 1 μM RB had practically no effect on Fv/Fm, 5 and 10 μM produced significantly larger Fv/Fm loss after 30 min, than 0 or 1 μM RB (Fig. 6a). When Chlorella cells were kept in darkness the Fv/Fm values showed a minor increase followed by a small decrease reaching ca. 97.5% of the initial PSII activity was kept after 0 min independent of the absence or presence of RB after 60 min (Fig. 6b).
To correct for the small changes of PSII activity in the dark the Fv/Fm data are also shown after normalization of the data obtained in the light to those obtained in the dark (Fig. 6c).
The changes in Fv/Fm are relatively small in Chlorella cells as compared to those obtained in isolated thylakoids, when the same irradiance and timescale of treatment is applied (Fig. 2).
However, after 60 min light treatment the enhanced decline of Fv/Fm is obvious in the presence of RB, especially in the lin- comycin treated cells (Fig. 6c). Although Fig. 6 clearly shows
Fig. 5 Fv/Fm image of Chlorella sorokiniana cells in the 24 well plates illuminated with green + white light (a, b) or kept in darkness (c, d) at 0 min (a, c) and after 60 min (b, d). Wells A1-3: 0 µM RB, A4-6: 0 µM RB + lincomycin, B1-3: 1 µM RB, B4-6: 1 µM RB + lin-
comycin, C1-3: 5 µM RB, C4-6: 5 µM RB + lincomycin, D1-3:
10 µM RB, D4-6: 10 µM RB + lincomycin. The color bar indicates the relative intensity of Fv/Fm (black: Fv/Fm = 0, magenta: Fv/Fm = 1)
the tendency of cells experiencing larger PSII activity loss in the presence of higher RB concentrations, the extent of these changes was also analyzed for statistically significant differ- ences. As shown in Fig. 7, illumination of the Chlorella cells in the absence of RB resulted in a significant loss of the maximal quantum yield of PSII after 60 min even in the absence of lin- comycin, showing that despite the relatively low light intensity photoinhibition of PSII has occurred. The PSII activity loss
was enhanced in the presence of lincomycin due to the lack of protein synthesis dependent repair. This effect was further enhanced when RB was present, as an external source of 1O2, and the extent of PSII activity loss was increasing practically linearly with increasing concentration of RB. In the absence of lincomycin there was a tendency of increased PSII activity loss in the presence of RB, but the effect was statistically significant only in case of 10 μM RB.
Fig. 6 Effect of RB on the maximum quantum efficiency of PSII (Fv/Fm) of Chlorella sorokiniana cells treated with green-white light (240 μmol photons m−2 s−1) (a), or kept in dark (b) for the indicated time periods. Panel c represents the ratio of Fv/Fm recorded during the light treatment and dark incubation experiment [Fv/Fm values in panel
(a) were divided by the values on panel (b)]. Cells were incubated in the presence of 0 µM (black), 1 µM (red), 5 µM (blue) or 10 µM (green) RB. Solid lines, cells with no lincomycin, dashed lines, cells in the presence of 300 μg/ml lincomycin
Fig. 7 Maximum quantum efficiency of PSII (Fv/Fm) of Chlorella sorokiniana cells treated with green-white light (240 μmol photons m−2 s−1) in the presence of 0 µM (black), 1 µM (red), 5 µM (blue) or 10 µM (green) RB for the indicated time periods (n = 9).
Closed bars, cells with no lincomycin, checkered bars, cells in the presence of 300 μg/
ml lincomycin. Different letters above the bars indicate statisti- cally different means, values sharing common letters are not significantly different from one another (p < 0.05)
Discussion
Externally produced 1O2 is capable of damaging PSII both in isolated thylakoids and intact Chlorella cells It is generally accepted that 1O2, as one of the highly reac- tive ROS forms, is involved in the process of photoinhi- bition (for reviews see Krieger-Liszkay 2005; Nishiyama et al. 2006; Vass 2011; Tyystjarvi 2013). However, the exact action mechanism of 1O2 is debated. The main topic of this debate is whether 1O2 can directly damage the PSII complex, or it can inhibit only the protein synthesis dependent repair of PSII. The idea that 1O2 is an elicitor of PSII photodam- age is supported by various lines of experimental evidence.
These findings demonstrate 1O2 production in light exposed isolated (Macpherson et al. 1993; Hideg et al. 1994a) and intact (Hideg et al. 1998; Rehman et al. 2013, 2016b) pho- tosynthetic systems. In addition, the correlation between the rate of photodamage and the rate of 1O2 production has also been clearly shown (Rehman et al. 2013; Hakkila et al.
2013, 2014; Bersanini et al. 2014; Sedoud et al. 2014). The findings that higher 1O2 production rates are accompanied in general with higher PSII photodamage rates do not nec- essarily prove that 1O2 is the cause of PSII damage, since in principle it may happen that photodamaged PSII cent- ers produce more 1O2 than the active ones. However, the effect of mutations that enhance or decrease photodamage in parallel with modifications of 1O2 production pathways in PSII, which suppress or enhance protective charge recom- bination routes, respectively (Fufezan et al. 2007; Rehman et al. 2013), provide strong support for the idea that 1O2 is indeed an agent that induces or mediates the damage of PSII.
This idea is supported also by the correlation of extreme light tolerance and decreased 1O2 levels in Chlorella ohadii (Treves et al. 2016).
The idea that 1O2 is a direct damaging agent of the PSII complex was challenged on the basis experiments, in which no PSII activity loss was observed when Synecho- cystis PCC 6803 cells were illuminated in the presence of RB, as an external 1O2 source, as well as chloramphenicol as protein synthesis inhibitor (Nishiyama et al. 2001, 2004, 2006). In contrast to these findings infiltration of RB into lincomycin treated intact tobacco leaves accelerated the loss of PSII activity and of the D1 protein (Hideg et al.
2007; Kovács et al. 2014) supporting the idea that external
1O2 can damage the structure and function of PSII. How- ever, the high concentration of RB which was applied in these leaf infiltration studies (1 mM and 100 μM, respec- tively) raised the possibility of unspecific damage by the high concentration of externally generated 1O2.
Our present data provides evidence that illumination in the presence of RB can indeed damage PSII activity even
when relatively low concentrations of RB (1–10 μM) are used, and this damaging effect can be ameliorated in thy- lakoids by adding histidine, which is a chemical scavenger of 1O2 (Fig. 3). These demonstrate that externally gener- ated 1O2 inactivates PSII and confirm the validity of earlier leaf infiltration studies in which much larger concentra- tions (100–1000 μM) of RB were used in (Hideg et al.
2007; Kovács et al. 2014). The damaging effect is obvi- ously more substantial in the isolated thylakoids (Figs. 1 and 2) than in the intact Chlorella cells (Figs. 5–7), but even in the latter case the enhancement of PSII activity loss is statistically significant and increases with increas- ing RB concentrations, especially in the presence of lin- comycin, which blocks protein synthesis dependent PSII repair (Fig. 7). 1O2 has a very short lifetime and travel distance in a cellular environment (Krasnovsky 1998; Sko- vsen et al. 2005), therefore it is unlikely that the 1O2, which is produced outside the cells could reach the thylakoid embedded PSII complexes in the chloroplasts. The ability of RB to penetrate inside the cells and reach chloroplasts has been shown in tobacco leaves (Kovács et al. 2014). RB can also influence intracellular processes in Synechocys- tis 6803 (Nishiyama et al. 2004) and Chlorella vulgaris (Dall’Osto et al. 2019) and this is also the case in Chlo- rella sorokiniana used in the present study. The reason for the weaker inhibition of PSII in the intact Chlorella cells in comparison to the isolated thylakoids should be related to the lower local RB concentration inside the chloroplasts in the algal cells as compared to the bulk medium, and/or the presence of efficient 1O2 scavenging processes in the intact system. This idea is supported by the observation that even though a large amount of 1O2 was produced at 1 μM RB concentrations in the bulk medium (Fig. 4) PSII activity loss was significant only in the higher concentra- tion range of 5–10 μM (Figs. 5, 6).
Similarly to the case of isolated thylakoids we have attempted to check the protective effect of histidine also in the Chlorella cells. However, the results were not conclusive (not shown) most likely due to the relatively small extent of PSII activity loss, which was observed as a result of illumi- nating Chlorella cells in the presence of RB (Figs. 6, 7). It is also possible that the penetration of histidine to the vicinity of PSII complexes inside the Chlorella cells, where it could exert its protective action, was limited. However, based on the strong protective effect of histidine in thylakoids, we can assume that histidine would ameliorate PSII activity loss in Chlorella as well, provided that it can reach the thylakoids inside the cells.
The loss of PSII activity by externally generated 1O2 is in full agreement with previous studies, which showed the impact of 1O2 in vitro can be related to the fragmentation of D1 protein, i.e. the scission of peptide bonds in the D1 protein (Lupínková and Komenda 2004; Miyao 1994), and
consequently inactivation of electron transport (Mishra et al.
1994), and also with the degradation of the D1 protein dur- ing illumination of RB infiltrated leaves (Hideg et al. 2007;
Kovács et al. 2014). In addition, mass spectrometry analy- sis of photodamaged PSII showed the presence of oxidized amino acid residues both at the acceptor and donor sides of the PSII reaction center complex, which demonstrates the damage of the D1 and D2 proteins by reactive oxygen spe- cies (Kale et al. 2017; Zhou et al. 2021).
Reasons for contradiction with earlier data
Considering the contradiction of results in the present study concerning the damaging effect of externally produced 1O2 with those of earlier studies by Nishiyama and coworkers in which no effect of RB induced 1O2 on PSII damage rate was observed (Nishiyama et al. 2004) it is important to clarify the possible causes of this disagreement. There are four main differences of the experimental conditions in the present study and in the previous investigation (Nishiyama et al. 2004): (i) Isolated spinach thylakoids and Chlorella sorokiniana cells vs. the cyanobacterium Synechocystis PCC 6803. (ii) Relatively weak (240 µmol photons m−2 s−1) green dominated light to specifically excite RB without signifi- cant excitation of Chl vs. strong illumination by white light (1500 μmol photons m−2 s−1). (iii) Lincomycin vs. chloram- phenicol as protein synthesis inhibitor in the present study.
(iv) PSII activity was assessed here by Chl fluorescence imaging to determine Fv/Fm vs. O2 evolution measurements by a Clark type oxygen electrode by Nishiyama et al. (Nishi- yama et al. 2004). In the following section we discuss the significance of these differences.
Experimental objects
In order to study the effect of externally produced 1O2 on PSII activity it has to be made sure that the added photo- sensitizer, in this case RB, can reach the vicinity of the tar- get, i.e. the PSII complex. This can be easily achieved in the aqueous suspension of isolated thylakoid membranes.
Therefore, we choose thylakoids as one of our experimen- tal object. The previous work (Nishiyama et al. 2004) was performed with the cyanobacterium Synechocystis 6803, which is undoubtedly a very useful and widely used model organism for photosynthesis and photoinhibition studies.
However, the lack of RB-induced PSII damage might have been caused by a limited penetration of RB inside the Syn- echocystis cells limiting the amount of 1O2 in the vicinity of the thylakoid membranes. Therefore, we choose the green alga Chlorella sorokiniana, since penetration of RB into Chlorella cells has been demonstrated in the closely related Chlorella vulgaris species (Dall’Osto et al. 2019). Since the damaging effect of RB-mediated 1O2 production was clearly
seen in both the isolated thylakoids (Figs. 1–3) and in the Chlorella cells (Figs. 5–7), one factor in the lack of detection of PSII activity loss in Synechocystis (Nishiyama et al. 2004) is probably the limited reach of RB into the vicinity of the thylakoid embedded PSII complexes in this cyanobacterium.
It is of note that some 1O2 was certainly produced by illumi- nation of RB in the Synechocystis cells as shown by the inhi- bition of psbA mRNA elongation, which is a crucial step of the D1 protein synthesis dependent PSII repair (Nishiyama et al. 2004). However, the solubility of RB in water is much better than in organic solvents, therefore the RB sensitized
1O2 can more easily reach the site of D1 synthesis, which takes place in ribosomes attached to the cytosolic surface of the thylakoid membrane (Tyystjarvi et al. 2001), than the hydrophobic inner parts of the thylakoids where most of the functional components of the PSII complex are located.
Illumination protocol
We used relatively low light intensity (240 μmol photons m−2 s−1) with a significant contribution in the green spectral range that excites specifically RB, without driving the photo- synthetic electron transport. As consequence we could avoid strong photoinhibition by photosynthetically active light and the damaging effect to PSII was induced mostly by the RB generated 1O2. In contrast, Nishiyama et al. (Nishiyama et al.
2004) used very strong (1500 μmol m−2 s−1) visible light, which in itself induced extensive photodamage of PSII, and combined this strong illumination with RB addition. As a consequence, the effect of RB addition on the photodamage rate was most likely relatively small, which could be left unnoticed. Actually, green illumination, which selectively excites RB was also used in the work by Nishiyama et al.
(Nishiyama et al. 2004), but only for assessing the 1O2 effect on the repair process (i.e. in the absence of protein synthesis inhibitor). It is a puzzle why this straightforward approach was not used for assessing the 1O2 effect on the photodam- age rate (i.e. in the presence of protein synthesis inhibitor)?
Protein synthesis inhibitors
To separate the processes of photodamage and repair of PSII intact cells have to be treated with a protein synthesis inhibi- tor. In photoinhibition studies the most popular protein syn- thesis inhibitors are lincomycin and chloramphenicol (Kodru et al. 2020). Their application is based on the assumption that they are not interacting with photosynthetic electron transport processes. Unfortunately, this is not the case for chloramphenicol, which has been shown to act as electron acceptor in PSI and reduce oxygen to reactive superoxide (Okada et al. 1991). More recently it was shown that chlo- ramphenicol accepts electrons not only from PSI, but also from PSII (Rehman et al. 2016a), and also that this effect
artificially enhances PSII photodamage via superoxide production in intact Synechocystis cells if chlorampheni- col is applied above 100 μg mL−1 concentration (Kodru et al. 2020). Therefore, in the present study lincomycin was used in contrast to the work by Nishiyama et al. in which 200 μg mL−1 chloramphenicol was applied (Nishiyama et al.
2004). Similarly, to the effect of strong light the presence of high chloramphenicol amount could enhance the photodam- age rate in the earlier study (Nishiyama et al. 2004) decreas- ing the chance to detect the RB effect.
PSII activity measurements
In the presence of RB illumination of the samples produce a large amount of 1O2, which can chemically react with, i.e. oxidize, proteins, lipids and other organic material in its environment. This oxidation results in O2 uptake that decreases the apparent O2 evolution rate detected by the Clark type electrode (see the decreased O2 rate in the pres- ence of 1 μM RB in Fig. 6). Since in the previous Synecho- cystis study (Nishiyama et al. 2004) 2 and 10 μM RB was used the modification of the O2 evolution rate must have been much larger than that we observed here, unless RB was washed out from the cells before the O2 evolution assay.
Although there is no indication for such a precaution in the Nishiyama paper this was most likely the case, otherwise the O2 evolution rate must have been very small even in the non-photoinhibited cells, which is not noted in the paper.
The main reason for using Chl fluorescence imaging method in our present study was that it made possible to perform all treatments and PSII activity measurements under exactly the same experimental conditions. In addition, the Chl fluo- rescence imaging method is not affected at all by the 1O2 induced O2 uptake problem. Therefore, using Chl fluores- cence imaging vs. O2 evolution measurements does not seem to be a crucial factor in the contrasting results.
Based on the above comparison of experimental condi- tions our conclusion is that the lack of detecting the RB- induced enhancement of PSII photodamage rate in the previous study (Nishiyama et al. 2004) was most likely the consequence of multiple effects: (i) The limited ability of 1O2 produced by RB to reach the thylakoid embedded functional components of the PSII decreased the chance of detecting PSII activity loss. (ii) The application of strong visible light instead of selective excitation of RB for the photodamage experiments together with using high chlo- ramphenicol concentration enhanced the photodamage rate, which could easily mask the relatively small enhancement of 1O2-induced PSII damage. In our study these technical problems were eliminated by selective excitation of RB and by the application of lincomycin as protein synthesis inhibi- tor, that does not interfere with PSII electron transport and made possibly the detection of 1O2-induced PSII activity
loss not only in the isolated thylakoids, but also in the intact Chlorella cells.
Conclusions
We provided clear experimental evidence that 1O2 when gen- erated by the externally added photosensitizer RB induces the loss of PSII activity both in isolated thylakoids and intact Chlorella cells, which are incapable of PSII repair. These data demonstrate that 1O2 when produced by an external source, outside of the photosynthetic apparatus, is capable of direct inactivation of PSII. Our results also provide support for the idea that 1O2 could act as an inducer of photodam- age in native photosynthetic systems when it is produced by chlorophylls and other pigments (Vass and Cser 2009), in agreement with the proposal that light absorption in the cata- lytic Mn cluster cannot be solely responsible for the inactiva- tion of PSII (Ohnishi et al. 2005), and other, Mn-independ- ent damage mechanisms should also be considered (Oguchi et al. 2009, 2011; He et al. 2015; Iermak et al. 2020).
Author contributions F Bashir performed most of the experiments. AU Rehman participated in the thylakoid experiments and supervised the work of F Bashir in the initial phase of the study. M Szabo directed the well plate-based imaging experiments. I Vass conceived the idea and wrote the manuscript with the contribution of all coauthors.
Funding Open access funding provided by ELKH Biological Research Center. The work was supported by the National Research, Develop- ment and Innovation Office (NKFIH K 116016). M.S. was supported by the Hungarian Academy of Sciences, MTA Premium Postdoctoral Research Program (grant ID: PREMIUM-2017–38) and by the National Research, Development and Innovation Office (grant ID: NKFIH FK 128977).
Data availability All data presented are available in the form of figures, and tables in the main text.
Declarations
Conflict of interest The authors have no conflicts of interest to declare that are relevant to the content of this article.
Open Access This article is licensed under a Creative Commons Attri- bution 4.0 International License, which permits use, sharing, adapta- tion, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http:// creat iveco mmons. org/ licen ses/ by/4. 0/.
References
Anderson JM (1981) Consequences of spatial separation of Photosys- tem 1 and 2 in thylakoid membranes of higher plant chlosroplasts.
FEBS Lett 124:1–10
Anderson JM, Chow WS (2002) Structural and functional dynam- ics of plant Photosystem II. Philos Trans R Soc B-Biol Sci 357(1426):1421–1430. https:// doi. org/ 10. 1098/ rstb. 2002. 1138 Aro E-M, Virgin I, Andersson B (1993) Photoinhibition of Photosys-
tem II. Inactivation, protein damage and turnover. Biochim Bio- phys Acta 1143:113–134
Barber J, Andersson B (1992) Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci 17:61–66
BareraDall’Osto SL, Bassi R (2021) Effect of lhcsr gene dosage on oxidative stress and light use efficiency by Chlamydomonas reinhardtii cultures. J Biotechnol. https:// doi. org/ 10. 1016/j. jbiot ec. 2020. 12. 023
Bersanini L, Battchikova N, Jokel M, Rehman AU, Vass I, Allahver- diyeva Y, Aro E-M (2014) Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in cyanobacteria. Plant Physiol 164(2):805–818
D’Alessandro S, Havaux M (2019) Sensing β-carotene oxidation in Photosystem II to master plant stress tolerance. New Phytol 223(4):1776–1783. https:// doi. org/ 10. 1111/ nph. 15924 Dall’Osto L, Cazzaniga S, Guardini Z, Barera S, Benedetti M, Man-
nino G, Maffei ME, Bassi R (2019) Combined resistance to oxi- dative stress and reduced antenna size enhance light-to-biomass conversion efficiency in Chlorella vulgaris cultures. Biotechnol Biofuels 12(1):221. https:// doi. org/ 10. 1186/ s13068- 019- 1566-9 Fischer BB, Hideg É, Krieger-Liszkay A (2013) Production, detec- tion, and signaling of singlet oxygen in photosynthetic organ- isms. Antioxid Redox Signal 18:2145–2162
Fischer BB, Krieger-Liszkay A, Eggen RIL (2004) Photosensitiz- ers neutral red (Type I) and rose bengal (Type II) cause light- dependent toxicity in Chlamydomonas reinhardtii and induce the Gpxh gene via increased singlet oxygen formation. Environ Sci Technol 38(23):6307–6313. https:// doi. org/ 10. 1021/ es049 Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, 673y
Nonell S, Wilson MT, Baker NR (2006) Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green®. J Exp Bot 57:1725–1734
Fufezan C, Gross CM, Sjödin M, Rutherford AW, Krieger-Liszkay A (2007) Influence of the redox potential of the primary quinone electron acceptor on photoinhibition in Photosystem II. J Biol Chem 282:12492–12502
Hakkila K, Antal T, Rehman AU, Kurkela J, Wada H, Vass I, Tyystjärvi E, Tyystjärvi E (2013) ΔsigCDE, a mutant of Synechocystis sp.
PCC 6803 having SigB as an only functional group factor, is vul- nerable to oxidative stress but resistant against photoinhibition.
Biochim Biophys Acta 54(11):1780–1790
Hakkila K, Antal T, Rehman AU, Kurkela J, Wada H, Vass I, Tyystjärvi E, Tyystjärvi T (2014) Oxidative stress and photoinhibition can be separated in the cyanobacterium Synechocystis sp. PCC 6803.
Biochim Biophys Acta 1837:217–225
He J, Yang WQ, Qin L, Fan DY, Chow WS (2015) Photoinactivation of Photosystem II in wild-type and chlorophyll b-less barley leaves:
which mechanism dominates depends on experimental circum- stances. Photosynth Res 126(2–3):399–407. https:// doi. org/ 10.
1007/ s11120- 015- 0167-0
Hideg É, Kálai T, Hideg K, Vass I (1998) Photoinhibition of photosyn- thesis in vivo results in singlet oxygen production. Detection via nitroxide-induced fluorescence quenching in broad bean leaves.
Biochemistry 37(33):11405–11411
Hideg É, Kós PB, Vass I (2007) Photosystem II damage induced by chemically generated singlet oxygen in tobacco leaves. Physiol Plant 131:33–40
Hideg É, Spetea C, Vass I (1994a) Singlet oxygen and free radical pro- duction during acceptor- and donor-side-induced photoinhibition.
Studies with spin trapping EPR spectroscopy. Biochim Biophys Acta 1186:143–152
Hideg É, Spetea C, Vass I (1994b) Singlet oxygen production in thy- lakoid membranes during photoinhibition as detected by EPR spectroscopy. Photosynth Res 39:191–199
Hirakawa K, Hirano T, Nishimura Y, Arai T, Nosaka Y (2011) Control of singlet oxygen generation photosensitized by meso-Anthryl- porphyrin through interaction with DNA. Photochem Photobiol 87:833–839
Iermak I, Szabo M, Zavafer A (2020) Special issue in honour of Prof. Reto J. Strasser - Analysis of OJIP transients during photoinactivation of Photosystem II indicates the presence of multiple photosensitizers in vivo and in vitro. Photosynthetica 58(2):497–506. https:// doi. org/ 10. 32615/ ps. 2019. 166
Jones LW, Kok B (1966) Photoinhibition of chloroplast reactions. I Kinetics and Action Spectra. Plant Physiol 41:1037–1043 Kale R, Hebert AE, Frankel LK, Sallans L, Bricker TM, Pospíšil
P (2017) Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II. Proc Natl Acad Sci 114(11):2988–2993. https:// doi. org/ 10. 1073/
pnas. 16189 22114
Keren N, Berg A, van Kan PJM, Levanon H, Ohad I (1997) Mecha- nism of Photosystem II photoinactivation and D1 protein deg- radation at low light: The role of back electron flow. Proc Natl Acad Sci USA 94:1579–1584
Kodru S, Rehman AU, Vass I (2020) Chloramphenicol enhances Photosystem II photodamage in intact cells of the cyanobacte- riumSynechocystisPCC 6803. Photosynth Res 145(3):227–235.
https:// doi. org/ 10. 1007/ s11120- 020- 00784-1
Komenda J, Sobotka R, Nixon PJ (2012) Assembling and maintain- ing the Photosystem II complex in chloroplasts and cyanobac- teria. Curr Opin Plant Biol 15:245–251
Kou J, Oguchi R, Fan D-Y, Chow WS (2012) The time course of photoinactivation of Photosystem II in leaves revisited. Photo- synth Res 113:157–164
Kovács L, Kálai T, Tandori J, Kós PB, Hideg É (2014) Assessing the applicability of singlet oxygen photosensitizers in leaf studies.
Photochem Photobiol 90:129–136
Krasnovsky AA (1998) Singlet molecular oxygen in photobio- chemical systems: IR phosphorescence studies. Mol Cel Biol 12:665–690
Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis.
J Exp Bot 56:337–346
Krieger-Liszkay A, Fufezan C, Trebst A (2008) Singlet oxygen produc- tion in Photosystem II and related protection mechanism. Photo- synth Res 98:551–564
Leisinger U, Rüfenacht K, Fischer B, Pesaro M, Spengler A, Zehnder AJB, Eggen RIL (2001) Plant Mol Biol 46 (4): 395–408. https://
doi.org/https:// doi. org/ 10. 1023/a: 10106 01424 452
Li L, Aro EM, Millar AH (2018) Mechanisms of photodamage and pro- tein turnover in photoinhibition. Trends Plant Sci 23(8):667–676.
https:// doi. org/ 10. 1016/j. tplan ts. 2018. 05. 004
Lima-Melo Y, Alencar VTCB, Lobo AKM, Sousa RHV, Tikkanen M, Aro E-M, Silveira JAG, Gollan PJ (2019) Photoinhibition of Photosystem I provides oxidative protection during imbalanced photosynthetic electron transport in Arabidopsis thaliana. Front Plant Sci 10:916. https:// doi. org/ 10. 3389/ fpls. 2019. 00916 Lingvay M, Akhtar P, Sebok-Nagy K, Pali T, Lambrev PH (2020) Pho-
tobleaching of chlorophyll in light-harvesting complex II increases in lipid environment. Front Plant Sci 11:14. https:// doi. org/ 10.
3389/ fpls. 2020. 00849
Lupínková L, Komenda J (2004) Oxidative modifications of the Pho- tosystem II D1 protein by reactive oxygen species: from isolated protein to cyanobacterial cells. Photochem Photobiol 79:152–162 Macpherson AN, Telfer A, Barber J, Truscott TG (1993) Direct detec- tion of singlet oxygen from isolated Photosystem II reaction cen- tres. Biochim Biophys Acta 1143:301–309
Matheson IBC, Lee J (1979) Chemical-reaction rates of amino-acids with singlet oxygen. Photochem Photobiol 29(5):879–881. https://
doi. org/ 10. 1111/j. 1751- 1097. 1979. tb077 86.x
Méndez-Hurtado J, López R, Suárez D, Menéndez MI (2012) Theoreti- cal study of the oxidation of histidine by singlet oxygen. Chem Eur J 18:8437–8447
Mishra NP, Francke C, van Gorkom HJ, Ghanotakis DF (1994) Destructive role ofsinglet oxygen during earobic illuminatin of the Photosystem II core complex. Biochim Biophys Acta 1186:81–90 Miyao M (1994) Involvement of active oxygen species in degradation
of the D1 protein under strong illumination in isolated subcom- plexes of Photosystem II. Biochemistry 33:9722–9730
Murata N, Allakhverdiev SI, Nishiyama Y (2012) The mechanism of photoinhibiton in vivo: re-evaluation of the roles of catalase, a-tocopherol, non-photochemical quenching, and electron trans- port. Biochim Biophys Acta 1817:1127–1133
Murata N, Nishiyama Y (2018) ATP is a driving force in the repair of Photosystem II during photoinhibition. Plant Cell Environ 41(2):285–299. https:// doi. org/ 10. 1111/ pce. 13108
Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of Photosystem II. Biochim Biophys Acta 1757:742–749
Nishiyama Y, Allakhverdiev SI, Yamamoto H, Hayashi H (2004) Sin- glet oxygen inhibits the repair of Photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp.
PCC 6803. Biochemistry 43:11321–11330
Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba H, Yokota A, Murata N (2001) Oxidative stress inhibits the repair of photoda- mage to the photosynthetic machinery. EMBO J 20:5587–5594 Nixon PJ, Michoux F, Yu J, Boehm M, Komenda J (2010) Recent
advances in understanding the assembly and repair of Photosys- tem II. Ann Bot 106:1–16
Ogilby PR (2010) Singlet oxygen: there is indeed something new under the sun. Chem Soc Rev 39(8):3181–3209. https:// doi. org/ 10. 1039/
B9260 14P
Oguchi R, Douwstra P, Fujita T, Chow WS, Terashima I (2011) Intra- leaf gradients of photoinhibition induced by different color lights:
implications for the dual mechanisms of photoinhibition and for the application of conventional chlorophyll fluorometers. New Phytol. https:// doi. org/ 10. 1111/j. 1469- 8137. 2011. 03669.x: 1- 14 Oguchi R, Terashima I, Chow WS (2009) The involvement of dual
mechanisms of photoinactivation of Photosystem II in Capsicum annuum L. plants. Plant Cell Physiol 50:1815–1825
Ohad I, Kyle DJ, Arntzen CJ (1984) Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton poly- peptides in chloroplast membranes. J Cell Biol 99:481–485 Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M,
Nishiyama Y, Murata N (2005) Two-step mechanism of photo- damage to Photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center.
Biochemistry 44:8494–8499
Okada K, Satoh K, Katoh S (1991) Chloramphenicol is an inhibitor of photosynthesis. FEBS Lett 295:155–158
Pospísil P (2009) Production of reactive oxygen species by Photosys- tem II. Biochim Biophys Acta 1787:1151–1160
Pospísil P (2012) Molecular mechanisms of production and scavenging of reactive oxygen species by Photosystem II. Biochim Biophys Acta 1817:218–231
Rehman AU, Cser K, Sass L, Vass I (2013) Characterization of sin- glet oxygen production and its involvement in photodamage of Photosystem II in the cyanobacterium Synechocystis PCC 6803 by histidine-mediated chemical trapping. Biochim Biophys Acta 1827:689–698
Rehman AU, Kodru S, Vass I (2016a) Chloramphenicol mediates superoxide production in Photosystem II and enhances its pho- todamage in isolated membrane particles. Front Plant Sci 7:5.
https:// doi. org/ 10. 3389/ fpls. 2016. 00479
Rehman AU, Szabó M, Deák Z, Sass L, Larkum A, Ralph P, Vass I (2016b) Symbiodinium sp. cells produce light-induced intra-and extracellular singlet oxygen, which mediates photodamage of the photosynthetic apparatus and has the potential to interact with the animal host in coral symbiosis. New Phytol 212(2):472–484 Renger G, Govindjee (1985) The mechanism of photosynthetic water
oxidation. Photosynth Res 6:33–55
Rinalducci S, Pedersen JZ, Zolla L (2004) Formation of radicals from singlet oxygen produced during photoinhibition of isolated light- harvesting proteins of Photosystem II. Biochim Biophys Acta 1608:63–73
Rinalducci S, Pedersen JZ, Zolla L (2008) Generation of reactive oxygen species upon strong visible light irradiation of isolated phycobilisomes from Synechocystis PCC 6803. Biochim Biophys Acta 1777:417–424
Schreiber U, Quayle P, Schmidt S, Escher BI, Mueller JF (2007) Meth- odology and evaluation of a highly sensitive algae toxicity test based on multiwell chlorophyll fluorescence imaging. Biosens Bioelectron 22(11):2554–2563. https:// doi. org/ 10. 1016/j. bios.
2006. 10. 018
Sedoud A, Lopez-Igual R, Rehman AU, Wilson A, Perreau F, Boulay C, Vass I, Krieger-Liszkay A, Kirilovsky D (2014) The cyanobac- terial photoactive orange carotenoid protein is an excellent singlet oxygen quencher. Plant Cell 26(4):1781–1791
Shoaf WT, Lium BW (1976) Improved extraction of chlorophyll-a and chlorophyll–b from algae using dimethyl-sulfoxide. Limnol Oceanogr 21(6):926–928. https:// doi. org/ 10. 4319/ lo. 1976. 21.6.
Skovsen E, Snyder JW, Lambert JDC, Ogilby PR (2005) Life-0926 time and diffusion of singlet oxygen in a cell. J Phys Chem B 109:8570–8573
Sonoike K, Terashima I (1994) Mechanism of photosystem-I photoin- hibition in leaves of Cucumis sativus L. Planta 194:287–293 Sonoike K, Terashima I, Iwaki M, Itoh S (1995) Destruction of
photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett 362:235–238
Suorsa M, Jarvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjarvi S, Paakkarinen V, Tikkanen M, Jansson S, Aro EM (2012) Proton gradient regulation-5 is essential for proper accli- mation of arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24(7):2934–2948. https://
doi. org/ 10. 1105/ tpc. 112. 097162
Telfer A, Oldham TC, Phillips D, Barber J (1999) Singlet oxygen for- mation detected by near-infrared emission from isolated Photo- system II reaction centres: direct correlation between P680 triplet decay and luminescence rise kinetics and its consequences for photoinhibition. J Photochem Photobiol B 48:89–96
Tiwari A, Mamedov F, Grieco M, Suorsa M, Jajoo A, Styring S, Tik- kanen M, Aro EM (2016) Photodamage of iron-sulphur clusters in photosystem I induces non-photochemical energy dissipation. Nat Plants 2(4):9. https:// doi. org/ 10. 1038/ nplan ts. 2016. 35
Treves H, Raanan H, Kedem I, Murik O, Keren N, Zer H, Berkowicz SM, Giordano M, Norici A, Shotland Y, Ohad I, Kaplan A (2016) The mechanisms whereby the green alga Chlorella ohadii, iso- lated from desert soil crust, exhibits unparalleled photodamage