ContentslistsavailableatScienceDirect
Acta Biomaterialia
journalhomepage:www.elsevier.com/locate/actbio
Full length article
Gelatin content governs hydration induced structural changes in silica-gelatin hybrid aerogels – Implications in drug delivery
Mónika Kéri
a, Attila Forgács
b,c, Vanda Papp
a,b, István Bányai
a, Péter Veres
b, Adél Len
d, Zoltán Dudás
e, István Fábián
b,c, József Kalmár
b,c,∗aDepartment of Physical Chemistry, University of Debrecen, Egyetem tér 1, H-4032 Hungary
bDepartment of Inorganic and Analytical Chemistry, University of Debrecen, Egyetem tér 1, H-4032 Hungary
cMTA-DE Redox and Homogeneous Catalytic Reaction Mechanisms Research Group, Egyetem tér 1, H-4032 Hungary
dNuclear Analysis and Radiography Department, Centre for Energy Research, Hungarian Academy of Sciences, Konkoly-Thege Miklós út 29-33, Budapest, H-1121 Hungary
eNeutron Spectroscopy Department, Wigner Research Centre for Physics, Hungarian Academy of Sciences, Konkoly-Thege Miklós út 29-33, Budapest, H-1121 Hungary
a rt i c l e i n f o
Article history:
Received 26 September 2019 Revised 22 December 2019 Accepted 10 January 2020 Available online 15 January 2020 Keywords:
Aerogel Hydration
Mechanism of drug release NMR spectroscopy SANS
a b s t r a c t
Silica-gelatin hybridaerogelsofvarying gelatincontent(from4 wt.% to24 wt.%)can beconveniently impregnatedwith hydrophobicactive agents(e.g.ibuprofen,ketoprofen)insupercriticalCO2 and used asdrugdeliverysystems.Contrastvariationneutronscattering(SANS)experimentsshowthemolecular levelhybridizationofthesilicaandthegelatincomponentsoftheaerogelcarriers.Theactiveagentsare amorphous,andhomogeneouslydispersedintheseporous,hybrid matrices.Importantly,bothfastand retardeddrug releasecanbeachievedwith silica-gelatinhybrid aerogels, andthe kineticsofdrug re- leaseisgovernedbythegelatincontentofthecarrier.Inthispaper,forthefirsttime,amolecularlevel explanationisgivenforthestrongcorrelationbetweenthecompositionandthefunctionalityofafam- ilyofaerogelbaseddrugdelivery systems.Characterizationofthe wetaerogelsbySANS andbyNMR diffusiometry,cryoporometryand relaxometryrevealedthatthedifferenthydrationmechanismsofthe aerogelsareresponsibleforthebroadspectrumofreleasekinetics.Low-gelatin(4–11wt.%)aerogelsre- taintheiropen-porousstructureinwater,thusrapidmatrixerosiondictatesfastdrugreleasefromthese carriers. Incontrast tothis, wetaerogelsofhigh gelatincontent(18–24 wt.%) show wellpronounced hydrogel-likecharacteristics,andawidegradualtransitionzoneformsinthesolid-liquidinterface.The extensiveswellingofthehigh-gelatinhybridbackboneresultsinthecollapseoftheopenporousstruc- ture,thatlimitsmasstransporttowardsthereleasemedium,resultinginslower,diffusioncontrolleddrug release.
StatementofSignificance
Developingnewdrugdeliverysystemsisakey aspectofpharmaceuticalresearch.Supercritically dried mesoporousaerogelsareidealcarriersforsmallmolecularweightdrugsduetotheiropenporousstruc- turesand large specificsurfaceareas.Hybrid silica-gelatinaerogelscan displaybothfastand retarded drugreleasepropertiesbasedonthegelatincontentsoftheirbackbones.Thestructuralcharacterization oftheaerogelsbySANSandbyNMRdiffusiometry,cryoporometryandrelaxometryrevealedthatthedif- ferenthydrationmechanismsofthehybridbackbonesareresponsibleforthebroadspectrumofrelease kinetics. The molecularlevel understanding of the functionalityofthese hybrid inorganic-biopolymer drugdeliverysystemsfacilitatestherealizationofquality-by-designinthisresearchfield.
© 2020ActaMaterialiaInc.PublishedbyElsevierLtd.
ThisisanopenaccessarticleundertheCCBY-NC-NDlicense.
(http://creativecommons.org/licenses/by-nc-nd/4.0/)
∗ Corresponding author at: University of Debrecen, Department of Inorganic and
Analytical Chemistry, 1 Egyetem tér, H-4032 Debrecen, Hungary. E-mail address: kalmar.jozsef@science.unideb.hu (J. Kalmár).
https://doi.org/10.1016/j.actbio.2020.01.016
1742-7061/© 2020 Acta Materialia Inc. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license.
( http://creativecommons.org/licenses/by-nc-nd/4.0/ )
1. Introduction
There are only a handful of studies published in the litera- turethat report the molecular-level investigation of the physico- chemical factors that govern the drug delivery properties of advancedmaterials [1–6].Evidently, noneof theavailable papers dealwiththe physico-chemicalinvestigation ofa promisingplat- formofdrugdeliverydevices:mesoporousaerogels.
Avarietyofnovelmesoporousinorganic(silica)andbiopolymer (polysaccharideandprotein)aerogelshaverecentlybeenprepared [7–12]. Aerogels dried under supercritical conditions are promis- ing platforms for oral andpulmonary drug delivery applications [13–15]. Novel administration routesand other biomedical appli- cations are also investigated [16–21]. Biocompatible aerogels are emergingvesselsformusculoskeletaldrugdelivery[22].
It is well-established that these open mesoporous materials canefficientlybe impregnatedwithactive ingredientsby adsorp- tiveprecipitationusingsupercriticalCO2.Thistechniqueyieldsthe highestpossibleloadings,andalsoensuresthattheactiveingredi- entsareconservedintheiramorphousformsinsidetheporouscar- riermatrices[23–26].Adsorptiveprecipitationalsoensurestheho- mogeneousdistributionoftheactiveingredientsinsidetheporous matrices, that is cooperatively dictated by the thermodynamics ofthe multilayer adsorption of the drugmolecules on the inner pore walls, and the intimate conditions of the pressure-drop in- ducedprecipitationofthedrugsfromsupercriticalCO2[24,27,28]. Ingeneral, therateofthereleaseofthe amorphousactiveingre- dientfrom the carrier is significantly faster than the dissolution ofthe(micro)crystallineformsofthesamedrug.Furthermore,the total drugconcentration is also elevated, because the solution is inequilibrium withthe amorphous solid, and recrystallizationis slow. Thus, this carrier strategy increases both the rateand the thermodynamicdrivingforce ofdrugdissolution,whichenhances bioavailability[18–20,24]. Remarkablesolubility increase has also beenachievedwithfatsolublevitaminswhenloadedintoaerogels [25,29].
Inordertooptimizethefunctionalityofanaerogelbaseddrug delivery system, it is necessary to develop feasible strategies for fine-tuningitsreleaseproperties.One possibilityistomodifythe surfaceofthecarrierandtuneitshydrophilicityand/orhydropho- bicity,which directly sets thestrength of interaction ofthe drug withthebackbone ofthe aerogel [21,30]. Preparing core-shell or coatedaerogel carriers has been proved to be a straightforward strategytoachieve retardedreleaseinthecaseofotherwiserapid releaseaerogel systems [20,31–33]. Coating silica-alginate hybrid aerogel with hydroxypropyl methylcellulose (HPMC) and Ca(II)- alginateresultsinca.3-timesslowerdrugreleasecomparedtothe uncoatedaerogel [31].The coating ofsilicaaerogel withEudragit introducespHcontrol.Comparedtotheuncoatedsilicaaerogelcar- rier,therateofdrugreleasefromthecoatedaerogelisca.5-times slowerinpH =1.0HCl solution,butitis intactinpH =7.2PBS [20,33].Anotherstrategy fortheoptimizationofcarrierfunction- ality is to implement aerogels of hybrid inorganic-organic back- bones.Thephysico-chemicalpropertiesofthesehybrids,andthus, theirreleasepropertiescaneasily betuned bychanging theratio oftheirconstituents[14,32,34–37].
Inourprevious publicationswehaveshown, thatsilica-gelatin hybrid aerogels of varying silica-gelatin ratios are versatile drug delivery systems [38–41]. These aerogels can be loaded with smallmolecularweighthydrophobic drugs(ibuprofen,ketoprofen, triflusal) up to 20 wt.% by adsorptive deposition from supercrit- ical CO2. The silica-gelatin platform can provide both rapid and sustained (retarded) release of active ingredients depending on the inorganic/organic ratio of the hybrid carrier matrix [40–42]. Ingeneral,therateandmechanismofdrugreleasefromaerogels is governed by the following factors in aqueous media [1,3,42].
1) Erosion and degradation of the carrier matrix. 2) Strength of the interaction between the drug molecules and the backbone of the carrier. 3) Hydration of the aerogel and the drug[43]. 4) Hydration induced deformation and/or swelling of the carrier matrix[3,40,44–46].
In a previous publication we reported the systematicinvesti- gation of the mechanism of releaseof ibuprofen and ketoprofen from silica aerogel and from silica-gelatin hybrid aerogel of mi- nor(4wt.%) gelatincontent[40].Ithasbeenestablished thatthe releaseofthe drugsis aboutone orderofmagnitudefasterfrom the hybrid aerogel of4 wt.% content than fromthe parent silica aerogel.The goalofthisprecedingprojectwasto understandthe molecularmechanismofthiseffect.Thatstudyhasrevealedthati) theinteraction ofthedrugs withthe aerogelcarriers isgoverned bytheirrespectiveprotonationstates;ii)theaerogelsdegradeinto d = 20–50 μm particles in water; iii) the porosity of the well- hydrated silica particles remains intact withopen pores;and iv) thesilica-gelatinhybridaerogel ofminor(4wt.%) gelatincontent behavessimilarlytotheparentsilicaaerogelunderreleasecondi- tions in all but one aspect, i.e. the hydrationof the backboneis much morefeasible dueto thepresence of gelatin. Thus,the re- markableincrease inthedrugreleaseratehasbeenattributedto the morepronounced hydrationof thehybrid silica-gelatinback- bonecomparedtosilica[40,47,48].
Thebiocompatibilityofsilica-gelatinaerogelshasbeentestedin vitroagainstmultiplecelllinesbyusingtheMTTassayandtime- lapsevide-microscopyimaging. The hybrid aerogelsproved to be non-cytotoxicinnature.Detailedresultsaregiveninourprevious publications[38,39].
The present study systematically investigates and compares the mechanisms of drug release from a series of hybrid silica- gelatin aerogels of a wide range of gelatin content from 4 wt.%
to 24 wt.%. Importantly, the carriers with low gelatin contents are rapid release systems, while aerogels withhigh gelatin con- tent show sustained release properties.Dry aerogels are charac- terized by scanning electron microscopy (SEM), N2 adsorption- desorptionporosimetryandsmall-angleneutronscattering(SANS).
The mechanismofwetting andhydrationisinvestigated by non- conventional nuclear magnetic resonance (NMR) methods, such as cryoporometry, diffusiometry and relaxometry, complemented withSANSmeasurements.Thestructuralinformationiscorrelated withhightime-resolutionreleaseexperiments,that areevaluated byappropriatemathematicalmodels.Finally,clearconclusionsare drawn betweenthe compositions,structures andhydrationprop- ertiesoftheaerogelsandthekineticsandmechanismofdrugre- lease. A mechanismis establishedfor thein-depth interpretation ofthefunctionalityofasystematicallydesignedaerogelbaseddrug deliveryplatform.
2. Experimental
2.1. Materialsandsolutions
Tetramethoxysilane (TMOS) was used as the silica precursor and it was obtained from Fluka. Food grade gelatin (Type A, 150kDa)waspurchasedfromDr.Oetker,offeringhighpurityand assured quality products. Methanol, acetoneand ammonium car- bonate ((NH4)2CO3) were purchased fromFluka. Ibuprofen (IBU) [(RS)−2-(4-isobutylphenyl) propanoic acid] and ketoprofen (KET) [2-(3-benzoylphenyl)propanoicacid]werepurchasedfromSigma- Aldrich.SupercriticalCO2wasproducedfrom99.95%puregas(Car- buros Metalicos SA). All aqueous solutions were prepared with Milli-Q water (Millipore). Other chemicals (HCl, NaOH, NaH2PO4, cyclohexaneandhexane)wereACSreagentgrade(Sigma-Aldrich).
2.2. Preparationofaerogels
Silica-gelatin hybridaerogel monoliths of varying gelatincon- tentweresynthesizedbyusingasol-gelprocess,asdescribedpre- viously[39–41].Hybridalcogelswereproducedbytheco-gelation of gelatin and TMOS. After gelation, a multiple step solvent ex- changeprotocolwascarriedoutusingpureacetoneintheend.The gelsweredriedwithsupercriticalCO2 at14MPaand80°Cusing themediumpressuretechnique[49].
Previousresultswithsilica-gelatinaerogelsverified,thattheto- talamountofgelatinishomogeneouslyincorporatedintotheaero- gel backbone, forming a hybridin each case[39–41]. The gelatin content ofthehybridaerogelswasmeasured bythermalanalysis tobe3.7,11,18and24wt.%,respectively[39–41].
Hybrid aerogels of gelatin contents higher than ca. 25 wt.%
were excluded from this study, because we experienced an ele- vatedriskofformationoflocalinhomogeneitiesinthebackbones, andsuchinhomogeneitiesoftenyieldinconclusivematerialsprop- erties.
2.3. Characterizationofdryaerogels
Scanning electronmicroscopy (SEM)images were recordedon a Hitachi S-4300 instrument (Hitachi Ltd., Tokyo, Japan).Aerogel shards,freshlysplitfromthemonolithswereimmobilizedwithsu- perglueandWood’smetal,andcoveredby5–6atomiclayerthick sputteredgoldconductivelayers.Typically,15kVacceleratingvolt- agewasused.
Thespecificsurfacearea,theporesizedistributionandthespe- cificporevolumeoftheaerogelsweremeasuredbyN2adsorption- desorption porosimetry (QuantachromeNova 2000e). All samples were degassed at 80 °C for24 h before themeasurements. Spe- cificsurfaceareaandporesizedistribution werecalculatedusing thestandardprotocolsofmultipointBETandBJHmethods,respec- tively.
2.4. Particlesizeofwetaerogels
Thesizedistributionofaerogelparticleswasmeasuredbyusing a hemocytometerandimage analysisafterwetgrindingthe sam- ples by a Potter-Elvehjem tissue grinder(10 min) andsonication (5 min) [40,47].Imageswere taken fromc =0.5 mg/mLsuspen- sionswitha1.3MPUSBmicroscopecamera.TheImageJsoftware wasusedforcalculatingthesizedistributionoftheparticles.
Additionally,thesizedistributionofaerogelparticleswasmea- suredbylaserdiffractionlightscattering(LDLS,MALVERNMaster- sizer2000)usingconventionalinstrumentsetupandoperation.
2.5. Zetapotentialofaerogelparticles
Aerogelswerewetgroundbyatissuegrinder(seeabove),and the zeta potential wasmeasured ata final aerogel concentration of0.1mg/mL onaMALVERN ZetasizerNanoZSinstrumentusing conventional instrumentsetup andoperation. Zeta potential was measuredbetweenpH=3.0andpH= 7.4.ThelowerpH-limit of theZetacell isspecifiedto bepH =3.0bythe manufacturer.The pH = 7.4medium used here wasthe same PBS buffer that was usedinthedrugreleaseexperiments.
2.6. NuclearMagneticResonance(NMR)methods
2.6.1. NMRcryoporometry
Waterandcyclohexanewereusedasprobeliquids.Ineachex- periment ca. 70mg dry, powderedaerogel was measured into a 5mm wideglassNMRtube.The amountoftheprobeliquidwas settoca.500μLinordertofullysaturatetheporesoftheaerogel,
plusintroduceaminorbulkphase.Afterhomogenization,thewet samples were stored for 24 h at room temperature before NMR measurements.
Melting and freezing experiments were performed on a 360 MHz NMR instrument equipped with a 5 mm direct QNP probe head and on a 400 MHz NMR instrument with an in- verse broadband probe head, both of which were cooled with driedairandBCU-05andBSCU-05coolingunits.TheCarr–Purcell–
Meiboom–Gill (CPMG) spin-echo pulse sequence was applied to eliminatethebroadsignalofthesolidphaseduringtheechotime (1.5 ms for water and10 ms for cyclohexane). Temperature was calibratedonglycol andmethanol[50].Experimentswere always started with melting, and subsequent freezing and melting cy- cleswereperformedinthegiventemperaturerange.Spin-echo1H spectra were recorded in 0.2–0.3 K steps keeping the sample at constanttemperaturefor5minbeforemeasurement.MestReNova 9.0softwarewasusedforspectrapostprocessing.
Themeltingandfreezingpointdepressionsofaliquidconfined in a small space, and the shape of the corresponding melting- freezinghysteresisloop (determinedby poregeometry)are given by the modified Gibbs_Thomson equations [51–53]. Model-free cryoporometryconstantswereapplied(Kc=30nmKforwater,96 nmKforcyclohexane).Furtherdetailsondataevaluationaregiven intheSupportingInformation.
2.6.2. NMRdiffusiometry
Ineachexperimentca.70mgdry,powderedaerogelwasmea- sured into a 5 mm wide glass NMR tube. The volume of added waterwas100,200and300μLindifferentexperiments,resulting inwater/aerogelmassratiosof1.3–4.3g/g.Thesuspensionswere mixed,sonicated,compactedbyhandandstoredfor24hatroom temperaturebeforeNMRmeasurements.
The self-diffusion coefficient of waterin wetaerogel samples weremeasuredwithastimulatedspinechopulsesequenceusing bipolargradient pulses(BIPLED) toavoideddy currents [54]. The experimentswereperformedonaBrukerAvanceII400NMRspec- trometerusingthestandardpulseprogramat298K.Thelengthof thegradientpulse(
δ
)wassetto2–3ms,whilediffusiontime() wasvariedbetween10and120msinordertostudyitseffecton theobserveddiffusioncoefficient(Dobs).Thegradientstrength(G) wasincreasedfrom0 to50 cm–1 Gauss in 32square-equidistant steps. Spectra were transformed andevaluated withMestReNova 9.0. Diffusion data were evaluated according to the expression:[55–58]
I=I0exp
−Dobs
γ
2(
−δ
/3) δ
2G2(1) When multiple diffusion domains are present in the sample eachdomainisrepresentedbyadifferentsingle-exponentialfunc- tionof agivenDobs value, andtheobserved decayisthe sumof thesefunctions[59,60].Inordertodeterminethenumberofdiffu- siondomains,datawereprocessedbyinverseLaplacetransforma- tion(Multi-ExponentialRelaxationAnalysis,MERA)onthebasisof theCONTINmethod.Datawerealsofittedwithsingle- andmulti- exponential functions by using the Levenberg–Marquardt least- squaresalgorithm(OriginPro8.6). Thegradientwascalibratedfor D2Otoobtaintherealdiffusioncoefficients[61].
2.6.3. NMRrelaxometry
Inthe relaxometry experiments,the water/ dryaerogel mass ratiowasincreasedfrom0to3.8g/g in12–15 steps.In eachex- periment ca.100 mg dry, powderedaerogel was introduced into a 10 mm glass tube and an aliquot of water was added. The wetaerogelsamplesweremixedandsonicated.Allsampleswere storedfor48h atroom temperatureinsealed vessels toachieve complete equilibration before NMR measurements. Experiments were performedwiththe same samplewettedin multiple steps,
andwithmultiplesampleswettedwithdifferentamountsofwa- ter.Theresultswereidenticalwithinexperimentalerror.
ThemeasurementofT2 transverserelaxationtimeswascarried out on a 20 MHz Minispec Bruker mq20 relaxometer using the classicalCPMGspin-echo pulsesequence.The echotimewasvar- ied between80 and 300 μs, andthe number of echoes was set toreach thetotal exponential decayof thesignal. The relaxation delaywasoptimizedaccordingtothelongitudinalrelaxationtime (T1),determinedpreviouslyusingtheinversion-recoverysequence.
Thelengthsofthe90°pulsewasdeterminedforeachsample,and variedbetween1.6–2.1μswithapulseattenuationof6dB.
Watercan be found indifferentrelaxationdomains ina sam- ple.Eachrelaxationdomaincontributestothemeasuredsignalby asingleexponentialdecay,andtheobservedsignal isthesumof thesedecays[55,62–66].Inordertodeterminethenumberofre- laxationdomains,dataweretransformedbyinverseLaplacetrans- formation (Multi-Exponential Relaxation Analysis, MERA) on the basisoftheCONTINmethod[67].Inlinewiththenumberofrelax- ationdomainsdeterminedbyMERA,primarydatawerefittedwith asingle-oramulti-exponentialfunctionbyusingtheLevenberg–
Marquardt least-squares algorithm (OriginPro 8.6). The T2 trans- verserelaxationtime andtheamplitudewere estimatedforeach domain.
2.7.SmallAngleNeutronScattering(SANS)
Dry, powdered aerogel samples were introduced into 2 mm- thick quartz cuvettes and measured without any pre-treatment.
Some sampleswere wettedeither withD2Oorwith a1:2 H2O–
D2Omixtureto awater/ dryaerogelmass ratioof3.3g/g.After homogenization, thewet sampleswere stored overnight atroom temperaturebeforeSANSmeasurements.
SANSexperimentswereperformedontheYellowSubmarinein- strumentatBudapest Neutron centre.This isa pin-hole type in- strumentwitha two dimensional neutrondetector. Twosample- to-detector distances (1.2 m and 5.4 m) and two wavelengths (4.38 ˚A and 10.23 ˚A) were used. The beam diameter was 8 mm.
Sampleswere measuredfor60–180minatroomtemperature. By modificationofthe wavelengthandsampledetectordistance,aQ rangeof0.008–0.4 ˚A–1 wascovered. Themomentum transfer(Q) isdefinedbythefollowingequation:
Q=4
π λ
sinθ
2 (2)
where
λ
is the wavelengthof the monochromatic neutron beam andϴisthe scatteringangle.The definitionofthe scatteringin- tensity(I)isasfollows:I
( λ
,θ )
=I0( λ ) η ( λ )
TVdd
(
Q)
(3)where
λ
is thewavelength ofthe monochromaticneutron beam, ϴisthe scatteringangle,I0 is the incomingneutron flux, is theunitsolidangle,η
(λ
)isthedetectorefficiency,TandVarethetransmissionandvolume ofthe sampleand dd(Q)isthemacro- scopicdifferentialcrosssection.Themacroscopicdifferentialcross sectionconveysstructuralinformationonthestudiedsystem.The measuredscatteringintensitywascorrected forsampletransmis- sion,empty cell scattering, solventscattering, detector sensitivity andbackgroundscattering.The nano-andmicrostructuralparam- etersofthescatteringobjectsaredeterminedfromthemathemat- icalanalysisofthecorrectedI(Q)curves.
ForawideQrangewhereboth,theGuinierandthePorodap- proximationscanbeusedfordifferentpartsoftheSANScurve,the combinationof both is valid. This is referred to asthe Beaucage
model,anditcoversthewholeQrange[68,69].
I
(
Q)
∼=Aexp−Q2Rg2 3
+B
⎧ ⎪
⎨
⎪ ⎩
erf
QR√g 6
3Q
⎫ ⎪
⎬
⎪ ⎭
−p
(4)
Rgistheaveragegyrationradius,pisthePorodexponent,and AandBarecoefficientsrelatedtothevolumeandnumberdensity ofthescatteringobjectsandtotheircontrast.ParametersAandB canbetreatedasadjustablescalingparameters.
Datafittingwasperformedbyusingleast-squaresalgorithmsin Igor Pro 6.1 software. Further details on the evaluation of SANS dataaregivenintheSupportingInformation.
2.8. Impregnationofaerogels
Dry, milled and sieved (dparticle < 125 μm) aerogel powders wereimpregnatedwithibuprofenandketoprofen.Theprocesswas realizedinanautoclaveinsupercritical CO2 by utilizingthetech- niqueof adsorptiveprecipitation.Impregnationwasperformedat 45 °C and 20 MPa in a stirred reactor for 6 h. The depressur- ization rate was0.2 MPa in order to avoid the crystallization of thedrugs,asdescribedpreviously[40,41].Drugcontentwasdeter- mined withRP-HPLCaftersoaking 5.0mgloaded aerogelsample in10.0mLmethanolfor2h.The ibuprofencontentoftheloaded aerogelsvariesbetween19and24wt.%,andtheketoprofencon- tentvariesbetween11and15wt.%,asgiveninTablesS1andS2in theSupportingInformation.Theprocessofimpregnationhasbeen discussedpreviously[40,41].
The impregnatedaerogelsamples were characterizedby X-ray diffraction (XRD)and infrared (FT-IR) spectroscopy methods. The results are in-line with previous, well-established observations, thatthedrugsareamorphousinsidethehybridaerogelcarrierma- trices[16,23,24,41].
2.9. Drugreleasetests
AcustombuiltfiberopticUV–Visspectrophotometerequipped withafastCCDdetector(AvaSpec-ULS-RS-TEC,Avantes)wasused to monitor the dissolution of the active agents from the loaded aerogels [70]. Dry, loaded aerogel milled, and sieved to uniform size (dparticle < 125 μm) was used in order to avoid any parti- clesize-relatedeffects.Loadedaerogelpowderwasweightedwith 0.01 mg precision into a carefully dried spectrophotometric cu- vette. Thecuvette wasthermostatedat37.0± 0.1°C.On-line de- tection wasstarted, and3.0 mL pre-heated release medium (pH 2.0HCl solutionor pH 7.4PBS)wasintroduced into the cuvette.
The suspension was stirred at 300 rpm by a 2 × 8 mm PTFE coatedmagneticstir-bar.Thedetectorwastypicallyoperatedwith 30msintegrationtimeand20subsequentspectrawereaveraged.
Absorbance changewasfollowed inthe 200–800nm wavelength rangein1.0nmstepsforatleast1000swithaminimumtimeres- olutionof1.0s.Asthedrugdissolvedfromtheaerogel,thecharac- teristicabsorbancesignalofeitherIBUorKETwasdetectedinthe suspension[9,40].Itisimportanttonote,thatthelight scattering of the aerogel suspension wastaken into correction by subtract- ing it from each recorded spectrum using the “dual-wavelength method” developed by LiuandZhu [71,72]. Thepublished proto- cols were implementedwithout any alterations.As a verification method,drugreleasewasalsofollowedbyHPLCforsustainedre- lease systems. The final concentration of the drugwas addition- ally measured in every experiment by HPLC by using a method describedinourpreviouspublication[41].Foreachsample,cumu- lativedrugreleaseisgivenasthepercentageofthetotalamount ofloadeddrugintheaerogel.Thedetailedconditionsofthedrug
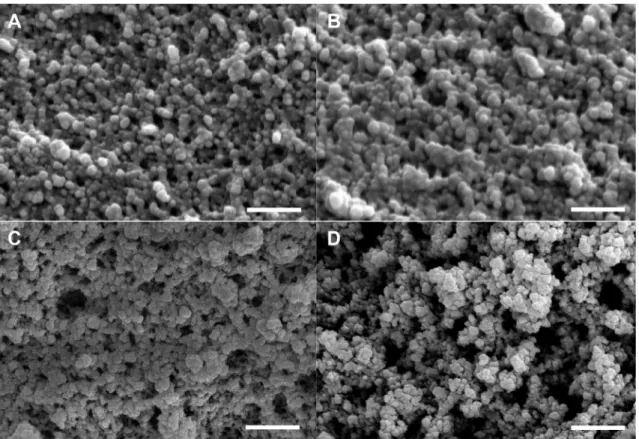
Fig. 1. Scanning electron micrographs (SEM) of a pristine silica aerogel (A) and silica-gelatin hybrid aerogels of 4 wt.% (B), 11 wt.% (C) and 24 wt.% (D) gelatin content. All scale bars represent 1.0 μm.
releasetestsarelistedinTablesS1andS2intheSupportingInfor- mation. For comparison, the solubilities ofthe amorphous forms ofIBUandKETarealsogiveninTablesS1andS2.Allexperiments wereperformedin3replicates.
Kineticmodelfittingwasperformedbasedonwell-established theories byusing arobust non-linear Levenberg-Marquardtleast- squaresalgorithm,asdescribedearlier[73].
3. Resultsanddiscussion
3.1. Morphologyandporestructureofdryaerogels
3.1.1. Scanningelectronmicroscopy
Micrographs of silica andsilica-gelatin aerogels are shown in Fig. 1in25k × magnification. Allstudied aerogelsarebuilt from primarysphericalblocks,thatisarchetypicaltosilicaaerogels.The size of these blocks is in the range of dsphere = 50 – 100 nm, andthissizeisindependentofthegelatincontentofthenetwork.
The fundamentalmorphology ofthehybrids is uniform,anditis similar tothat ofpure silica aerogels. However,both thenumber andthe sizeofmacrospores increasesystematically withincreas- inggelatincontent.Thread-likemotifsarealsovisibleforaerogels with24wt.%gelatin. Thesefindings suggest systematicstructural changesinthehybridsduetotheincorporationofgelatinintothe silicanetwork.
3.1.2. N2adsorption-desorptionporosimetry
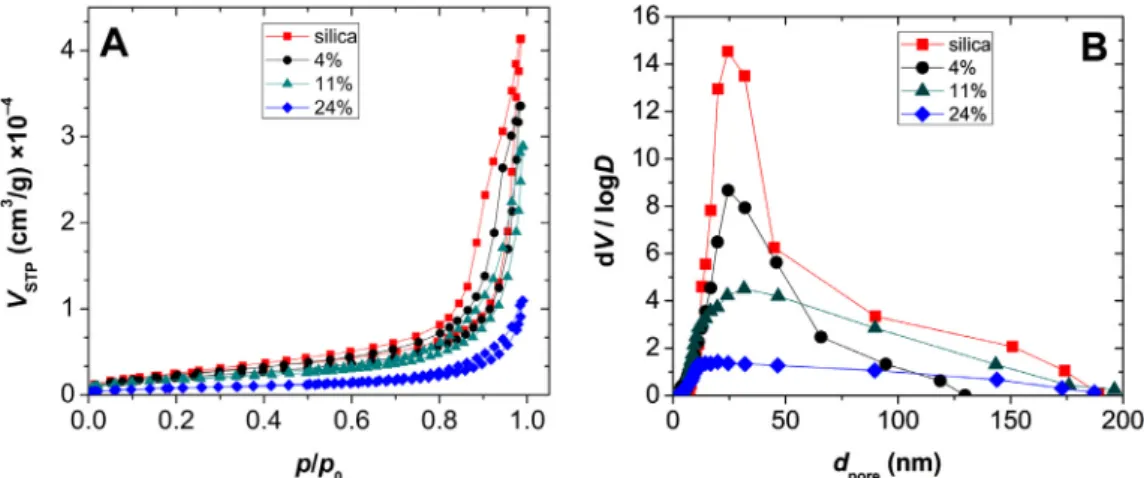
Representative N2 adsorption-desorption isotherms are shown inFig.2Afordrysilicaandsilica-gelatinaerogels.Alloftheexper- imentalhysteresiscurvesareIUPACtypeIV(H2),whichischarac- teristic formesoporousmaterials. The steeprise oftheisotherms atp/p0=1indicatesthepresenceofmacropores[74].Thespecific surfacearea (SBET) ofthe hybridaerogelsis between799m2 g–1 and 285 m2 g–1 and it systematically decreases with increasing
Table 1
Textural properties of silica and silica-gelatin hybrid aerogels derived from N 2gas adsorption-desorption porosimetry data (cf. Fig. 2 ). Mean ±SD values represent 3 replicate measurements.
S BET(m 2/g) a V p(cm 3/g) b d pore(nm) c C -constant
silica 812 ±61 6.20 (198 nm) 32 89
4 wt.% gelatin 799 ±72 4.95 (161 nm) 25 73 11 wt.% gelatin 627 ±60 4.48 (205 nm) 32 92 18 wt.% gelatin 416 ±46 2.31 (213 nm) 17 87 24 wt.% gelatin 285 ±32 1.69 (194 nm) 20 85
a BET specific surface area.
bTotal specific pore volume (upper threshold of pore diameter included in the calculation).
cMean pore diameter: estimated at the maximum of the distribution curve.
gelatin content (Table 1). The pore size distribution curves of theaerogels calculatedby the BJH methodare shown inFig. 2B.
In order to highlight the differences between the distribution curves,the x-axis isshown on thenormal scale, andnot on the logarithmicscale.Theporesizedistributioniswiderandthecon- tribution ofthe larger pores ismore significant at highergelatin content.Thetotalspecificporevolume(Vp)correspondingtodpore
<200nmsystematicallydecreaseswithincreasinggelatincontent (Table 1). Unfortunately, pores larger than 200 nm are excluded fromtheN2porosimetryanalysis,asaconsequenceoftheworking principle of the technique. Thus, the Vp value is representative only when the macropore contribution is small. This is further discussedinconnectionwithNMRcryoporometryinSection3.2.3. According to the combined SEM and N2 porosimetry results, thecontributionofmacroporesissignificant athigh(18–24wt.%) gelatincontents.Theincreasing amountofincorporatedgelatinin thebackboneresultsintheopeningandlooseningofthedryaero- gelmatrix.Thecontributionofmicroporeswasestimatedforeach aerogelby using thet-plotmethod,based on thede Boer model
Fig. 2. Nitrogen adsorption-desorption isotherms (A) and BJH pore size distribution curves (B) of silica and silica-gelatin hybrid aerogels of different gelatin content (given in the legend as wt.%).
Fig. 3. Small angle neutron scattering (SANS) curves of silica and silica-gelatin hybrid aerogels measured in their dry states (A) and when completely hydrated (B) by 1:2 H 2O:D 2O mixture (3.3 g liquid / g dry aerogel). Solid lines represent data fitting to the Beaucage model ( Eq. (4) ). Estimated structural parameters for the dry aerogels are given in Table 2 . (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
ofstatisticalthickness[75].The resultsindicatethat thereareno porespresentbelowdpore=2nminanyoftheaerogels.
3.1.3. MorphologyofdryaerogelsbySANS
Nanometer sized materialinhomogeneities (scattering objects) thathave neutronscattering length densitiesdifferent fromtheir surroundings yield meaningful SANS curves. These scattering curves carry information on the size, spatial distribution, shape andsurfacemorphologyofthescatteringobjects.Theprimarydif- ference inneutron scatteringlength densities in drymesoporous aerogels,i.e.the contrast,is betweenthesolid backboneandthe airfilledpores [52,76,77].Inthecaseofhybridmaterials,thedif- ferentcomponentsofthebackbonecanalsogivedistinctcontrasts.
Small angle neutron scatteringcurves measured for drysilica andsilica-gelatinaerogelsareshowninFig.3A.Allofthesecurves can be adequately fitted with the Beaucage model (Eq.(4)). The averagegyrationradius(Rg) andthePorodexponent(p) werees- timatedforall dryaerogels(Table 2).The value ofthe Porodex- ponents are between 3 and 4 for silica aerogel and for hybrids oflow gelatin content (4 wt.%). Such Porod exponents are char- acteristicfor surfacefractals. The value ofthe Porod exponent is ca.4forhybrid aerogelswithmoderateandhighgelatincontent (11wt.%–24wt.%),meaningthattheboundarybetweenobjectsof differentcontrastsissharpandsmooth[69].Thiscanbeattributed tothehighnumberofmacropores.Formacropores,theareaofthe porewallsis highandtheir apparent curvature islow compared tomicroporesandsmallmesopores.Inlinewiththisexplanation,
Table 2
Values of the Gyration radius ( R g) and the Porod exponent ( p ) of dry and hydrated silica and silica-gelatin aerogel samples. Structural parameters were estimated by fitting small angle neutron scattering (SANS) curves (cf. Figs. 3 A and 9 ) with the Beaucage model ( Eq. (4) ).
dry hydrated (D 2O)
silica R g= 36.8 ˚A R g= 31.9 ˚A
p = 3.28 p = 3.87
4 wt.%
gelatin R g = 37.3 ˚A R g = 30.3 ˚A
p = 3.44 p = 3.97
11 wt.%
gelatin
R g = 36.4 ˚A R g = 28.2 ˚A
p = 3.98 p = 5.15
24 wt.%
gelatin
R g = 54.0 ˚A R g: N/A
p = 4.22 p = 2.71
thegyration radiusis thehighestinthecaseof theaerogelwith thehighestgelatincontent.Thus,theresultsoftheSANSmeasure- ments areinexcellent agreement withthoseofthe SEMandthe N2porosimetry.
3.1.4. HomogeneityofthehybridbackbonebySANS
The primary experimental observation is that the scattering curvesofthedrysilicaandsilica-gelatinaerogelsdonotshowdis- tinctfeatures,i.e.thereisnosecondarycontrastinthecaseofthe hybridmaterials.Thesimplestexplanationforthisphenomenonis toassumethatthereisnospatialheterogeneityinthesolidsilica- gelatinbackbones[78].Inotherwords,thehybridbackbonesscat-
ter as homogeneous materials, andcontrast is the sole resultof porosity.
In order to verify this theory,contrast-variation SANS experi- ments were performed.Silica andsilica-gelatinaerogelswere hy- drated (3.3 g liquid / g aerogel)with 1:2 H2O:D2Omixture. The scatteringlengthdensityofthismixtureisca.equivalentwiththat ofsilica[77].Thus,theresultingscatteringcurveofhydratedsilica aerogel is practically equivalent to the background. Interestingly, almost identical, uninformative scattering was measured for the hydrated hybrid aerogels aswell, regardless of their gelatin con- tent (Fig. 3B). The most feasible explanation is that the contrast ofthesilica-gelatinmatrixapproximatelymatchesthatofthepure silica.Thisisastrongindicationthatthehybridizationofthesilica- gelatinbackboneishomogeneousatthemolecularlevel,i.e.gelatin doesnotforma“layer” onpuresilica,orshowsanyspatialprefer- ence[78].
3.2. Porestructureandmorphologyofhydratedaerogels
Waterreadilyinteractswithboth silicaandsilica-gelatinaero- gels.Twoconcertedprocessestakeplace.Thesolidbackboneishy- drated, and in the same time, the monolithic structure partially disintegrates to yield microparticles [40,47]. Hydration can take different extents based on the composition of the aerogel back- bone,whichcanleadtothealterationofthemorphologyandthe porestructureofthewetaerogel.Thedisintegrationoftheaerogel monolithsistheresultofbreakingthebondsbetweentheprimary buildingblocksinthesolid networkduetothesurfacetensionof waterandminorhydrolyticreactions. Asaresultofthetwo cou- pledprocesses,aerogelmicroparticlesforminwater.Theporenet- workofthesewetparticleseitherretainstheoriginalstructureof thedrysolids,orgetsdistortedduetotheextensivehydrationof thebackbone.
Thekineticsofhydrationofthesilicaandsilica-gelatinaerogels isfast,andcompleteinafewsecondswhenthesolidmatricesare floodedwithwater.
3.2.1. Particlesizeofhydratedaerogels
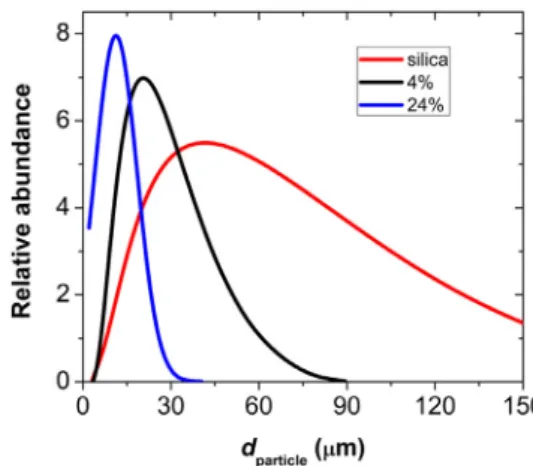
Particle size distribution of silica and silica-gelatin aerogels were measured afterwet grindingthesamples usinga standard- izedprotocol(cf.Section2.4.).Thisprotocolensuresthatthediffer- enceinparticlesizeisexclusivelyduetothedifferentcomposition oftheaerogels,whichinturngovernstheirhydrationanddegrada- tion.Sizedistributioncurvesmeasuredbyimageanalysisofmicro- graphs andbylaser diffractionlight scattering(LDLS)agreewell;
the maximum difference in peak position is 12% among parallel measurements.
Boththe position ofthe maximumand thewidth ofthe par- ticle size distributioncurve decreasesystematically by increasing gelatincontent(Fig.4).Inhybridaerogels,thecovalent silicanet- workisdisruptedbytheincorporationofgelatinproteinmolecules thatareboundtoeachotheronlybyweaksecondaryforces.These secondarybondseasilybreakuponthehydrationofthebackbone.
Thus,highgelatincontentfacilitatesthedegradationoftheaerogel monoliths asa consequenceofthefacilehydrationoftheprotein molecules.
3.2.2. Zetapotentialofaerogelparticles
Besides the size of the hydrated aerogel microparticles, their charge has a fundamentalinfluenceon the drugdelivery proper- ties, as well, because it controls the strength of the interactions betweentheaerogelbackboneandthedrugmolecules.
Thezetapotential(EZ)ofhybridaerogelparticleswasmeasured betweenpH=3.0andpH=7.4(Fig.5).The zetapotentialofsil- icaaerogelis+6± 1mVatpH=3.0, and−28± 2atpH=7.4.
The isoelectricpoint ofsilica isatca.pH=3.5, whichisingood
Fig. 4. Particle size distribution of wet silica and silica-gelatin hybrid aerogel parti- cles measured by laser diffraction light scattering (LDLS). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5. Zeta potential ( E Z) of silica and silica-gelatin aerogel microparticles as func- tion of pH.
agreementwithpreviouslyreportedvalues[79].Thezetapotential ofthehybridaerogelssystematicallyincreaseswiththeir increas- ing gelatincontent atall pHvalues. The proportionalitybetween EZ andgelatincontent iswell-expressed atacidicpH(positiveEZ values),butnot atneutralpH (negativeEZ values).The hybridof 24wt.% gelatinhasa maximum EZ of+22± 3 mVat pH= 3.0 and−22±2mVatpH=7.4.TheisoelectricpointofaqueousType Agelatinisreportedtobeatca.pH=5.5[80],that isconsistent withthepresentresults.
3.2.3. NMRcryoporometry
The theory and application of NMR cryoporometry are dis- cussed in detail by Strange et al., Petrov and Furó [51,52]. The primary information in NMR cryoporometry is the melting and freezingpointdepressionsofprobeliquiddropletsconfinedinside micro-,meso– and/or macroporous solid networks.This informa- tioncanbetranslatedtodropletsizedistribution,whichisequiv- alenttoporesizedistributionwhentheliquidcompletelyfillsthe poresofthenetwork[53].Inthiscase,thedominatingshapeofthe poresisalsoreflectedintheprimarymelting– freezinghysteresis curves.
First,NMRcryoporometrymeasurementswereconductedwith wet silica andsilica-gelatin aerogels containingenough water to completely fill their pores. In the case of the silica aerogel, the cryoporometrycurves indicatethat thepore structure of thehy- dratedaerogelisnearlyidenticaltothatofthedrygel.Thecalcu- latedporesize distributionisalmostincompleteagreementwith
Fig. 6. NMR cryoporometry of silica-gelatin hybrid aerogels in cyclohexane. The filling of the pores is complete, and the amount of the bulk-liquid and the pore-liquid is ca. equivalent. Panel A: melting – freezing hysteresis curves. Panel B: Volume equivalent pore size-distribution curves calculated from the data in panel A. The calculation is based on the modified Gibbs-Thomson equations.
theonemeasured byN2porosimetryinthedrystate[40,47].The generalconclusion isthat duringthe hydrationofsilica aerogels, themonoliths disintegrate intomicroparticles (cf.Fig. 4),butthe silicanetworkandtheporestructureofthemicroparticlesremain intact,atthesametimetheyarefilledwithwater[40,47].
Interestingly, no meaningfulNMR cryoporometrycurves could be recorded for fully hydrated silica-gelatin hybrid aerogels, re- gardless of their gelatin content. A melting – freezing hystere- sis loop was present in the case of the aerogel with the low- est(4 wt.%)gelatincontent,butwell-expressed stepswereabsent [40,47]. In the case of the hybrids with higher gelatin content, practically no melting and freezing point depressions were de- tected.Thiscanbeattributedtothestronghydrationofthehybrid backbonewhich alters the pore structure and yields a hydrogel- likematrix[81].Unfortunately,the extentofthealteration ofthe porenetworkcomparedto thedrystatecannot be deducedfrom thesecryoporometrymeasurements.
In order to prove that the observed collapseof the pore net- workcan beexclusively attributedtothehydrationofthe hybrid aerogels,another probe liquidwasimplemented forcryoporome- try[81].Cyclohexaneisa liquidthatwell solvatesbothsilicaand gelatin, and it is a widely used probe for cryoporometry. Repre- sentative melting – freezing curves and corresponding pore size distribution plots are shown in Fig. 6. The 1:2 ratio appearance ofthemelting– freezinghysteresissuggeststhedominatingpres- enceofsphericalporesinallaerogels[51].Theporesizedistribu- tioncurvescalculatedfromcyclohexaneNMRcryoporometryarein excellent agreement withthose measured by N2 porosimetry for the dryaerogels (cf. Figs. 2B and6B). This means that the pore structuresofthedryaerogelsarepreservedupon solvationbycy- clohexane.The mesopore andthe macropore contributions were calculatedfrom thesize distribution curvesin Fig. 6B.Total spe- cificporevolume (VpNMR) was calculatedfromthe primary NMR cryoporometry data, specifically from the height of the step as- sociated withthefreezing/meltingprocess (see integral values in Fig.6A).Theratiooftheheightofthisstepandthemaximumin- tegralvaluemeasuredatthecompletemeltingoftheprobeliquid isdirectlyproportional to the ratioofthe liquidfilling thepores andthetotalliquidcontentofthesample.Thiscalculationmethod isdescribed in our earlierpublication [40]. The estimated struc- turalparameters are giveninTable3 foreach silica-gelatinaero- gel.Theresultssupporttheconclusionsdrawnfromtheevaluation oftheSEMpicturesandtheN2 porosimetrydataforthedryaero- gels,i.e.macroporecontributionsignificantlyincreaseswiththein-
Table 3
Structural parameters of silica-gelatin hybrid aerogels estimated from primary and derived NMR cryoporometry data (cf. Fig. 6 ).
V pNMR(cm 3/g) a %mesopore b %macropore b
4 wt.% gelatin 3.4 ±0.5 38 62
11 wt.% gelatin 4.5 ±0.5 < 10 > 90 24 wt.% gelatin 4.3 ±0.5 < 10 > 90
a Total specific pore volume.
bCumulative volume equivalent mesopore and macropore contributions derived from pore size distribution.
creasinggelatincontentofthehybridaerogels.Importantly,thees- timatedspecificporevolumes(VpNMR)ofthesilica-gelatinaerogels areapproximatelythesameregardlessoftheirgelatincontent.This is only an apparent contradiction with the N2 porosimetrydata, where Vp decreases with increasing gelatin content (cf. Table 1).
Unfortunately,theVpvalueisnotrepresentativewhenthemacrop- orecontributionishigh,becauseporeslargerthan200nmareex- cludedfromtheN2 porosimetryanalysis.Thus,itismorerealistic toaccept theVpNMR valuesmeasuredincyclohexanetorepresent thespecificporevolumesofthepristinesilica-gelatinaerogels.
As a summary, NMR cryoporometry measurements verify the presenceofastrong,specificinteractionbetweenwaterandsilica- gelatinhybridaerogels.Thisinteractionresultsinsuchalterations and distortions of the pore networks that do not take place in othersolvents.
3.2.4. NMRdiffusiometry
The observed self-diffusion coefficient (Dobs) ofa liquidis al- teredcomparedtothebulk phase,whentheliquidisconfinedin theporesofasolidnetwork.Theapparentself-diffusioncoefficient isnotindependentofthelengthoftheobservationtimeofthedif- fusion experiment() in confinement.At limiting-shortobserva- tiontimes,themoleculestravelshorterdistancesthanthesizeof theporesanddonotcollidewithwalls.Inthiscase,theobserved self-diffusion coefficient is equivalentto that of measured in the bulkphase[59,60].Atlongerobservationtimes,collisionswiththe wallsreducethe effectivedistancecoveredbythe molecules,and theapparentself-diffusioncoefficientislowered.Therefore,asuffi- cientlyhighobservationtimeshouldbechosenifthegoalofdiffu- siometryistoobtaininformationontheconfinementoftheliquid andonthepermeabilityofthesolidnetwork[55–58,60].
Itispossiblethatwaterisfoundinmultiplediffusiondomains (e.g.intheprimary hydrationsphereandasa bulkphase)inthe
Fig. 7. NMR diffusiometry of hydrated silica-gelatin hybrid aerogels. Two diffusion domains are present in aerogels of lower gelatin content (4–11 wt.%) at low water content ( < 2.0 g/g), and this is reduced to 1 diffusion domain at high water con- tent. Only one diffusion domain is present in the hydrated 24 wt.% gelatin aerogel, regardless of its water content. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
samesample.Ifwatermoleculesinthedifferentdomainsexchange slowly with each other on the timescale of diffusiometry, more than oneapparent self-diffusioncoefficientsare measuredforthe samesample[55–58].
Whentheporesofsilicaaerogelarecompletelyfilledwithwa- ter, only one characteristic diffusion domain is present [47]. The value ofthesingleobservedself-diffusioncoefficientissetbythe ratioofconfinedwaterintheporesandbulkwater.Thisindicates that i) the primary hydrationsphere of the silicanetwork is not largeenoughtoformanindependentdiffusiondomaininthesam- ple,andii)the interchangeofwaterintheporesandinthebulk phase is facile. Accordingly, the pore network of hydrated silica aerogelisopenandpermeable[47].
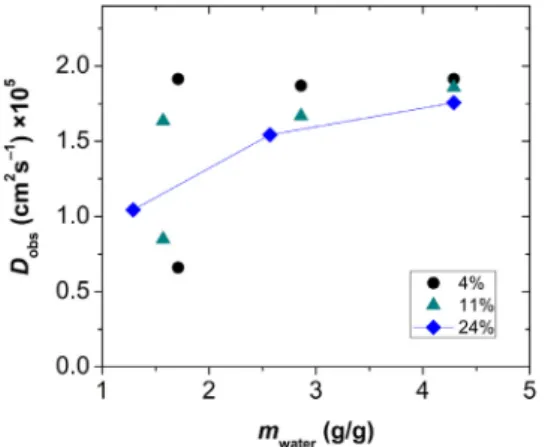
The apparent self-diffusion coefficient of water (Dobs) in hy- drated silica-gelatinaerogelswasmeasured by PGSE NMR exper- iments.The effectof2 variableswastested:i) thewatercontent ofthehybridaerogelsamples,andii)theobservationtimeofthe diffusionexperiments[82,83].First,itwasestablishedthattheDobs valuesareindependentoftheobservationtimeat≥40msinall hydratedsilica-gelatinaerogels(cf.Fig.S1intheSupportingInfor- mation).Thereafter, thewatercontentofeach samplewasvaried anditseffectonDobswasdeducedbyusinghighobservationtimes forthemeasurements.
Two diffusion domains were detected for aerogels of lower gelatin content (4–11 wt.%) at water contents below 2.0 g/g (Figs.7 andS1). Theslowerdiffusion domainischaracteristic for water molecules in the primary hydration sphere of the hybrid backbone,[48]whilethefasterdomainrepresentswatermolecules moving more freely inside the pores.The 2 domains are in slow exchange with each other,and theslower diffusion domain van- isheswhenthewatercontentofthesampleisincreased.Afterthe saturation of thehydrationsphere, mostof thewater diffusesin thepores andthisphasedominatestheNMRsignal.Interestingly, only1diffusiondomainispresentinthehydrated24wt.%gelatin aerogel, regardless of the water content of the sample (Figs. 7 and S1). This single Dobs value systematically increases with in- creasingwatercontent, corresponding toa lessandlesscompact non-porousstructure.Hydrogelstypicallyshowthisbehavior[84]. Finally, at highwater content, all hydrated silica-gelatin aerogels display approximately the same, single apparent self-diffusion coefficient for water, independently ofthe gelatin content ofthe matrix (Fig. 7). The highest observed self-diffusion coefficient of waterisca.2.0× 10–5 cm2s–1 inthe aerogelsamples,whilethat is2.3×10–5cm2s–1inthepurebulkphaseat25°C[61].
Theseresults indicate,that hybrid aerogelswithlower gelatin content retain a more or less permeable pore structure when treatedwith water, but hydrated 24 wt.% gelatin aerogel attains hydrogel-likecharacteristicsevenatlowwatercontent.
3.2.5. NMRrelaxometry
The theory for interpreting 1H NMR relaxation data used to describe the wetting mechanisms of porous solids is adopted from prior literature [62,63,65,67]. The relaxation rates of pro- tons in water molecules heavily depend on the localization and theenvironmentofthemoleculesinthesample.Generally,water moleculesinthehydrationsphere(adsorptionlayer)ofasolidma- trixandmoleculesconfinedinsmallporesrelaxfasterthanwater moleculesinthebulk liquid.The wettingmechanismofa porous solidcanbededucedbytitratingitwithwaterandmeasuringthe T2 relaxationtimeasfunctionofthewatercontentofthesample [55,62,63,65].
Inthe caseofhydrophilic porous solids,watermolecules first adsorb onthe surfaceformingmono- andmultilayers.Waterad- sorbedonahydrophilicsurfaceischaracterized bya shorttrans- verse relaxation time (T2s) compared to bulk water (T2b). When moleculesinthese2domainsexchangeonthetimescaleofrelax- ometry,theobservedT2 relaxationtimecanbe givenasthecom- binationofthe2limitingrelaxationtimes:
1 T2 = Vs
V0× 1 T2s+Vb
V0 × 1
T2b (5)
whereV0isthetotalvolumeofwaterinthesample,VsandVbare thevolumesofwateronthesurfaceandinthebulkphase,respec- tively(V0=Vs +Vb),andT2 istheobservedtransverserelaxation time.
Whentheamountofwaterisincreasedinthesolidsample,the filling ratioof the pores is higher. At higherfilling ratios, multi- ple quasi-bulk regions (puddles)can formatop the primary wa- ter layer, e.g. in the corners of concave pores. If these puddles are large, the exchange of water molecules between the quasi- bulkphaseandtheprimary layercanbeslowenoughfortheob- servationofmultipleT2 relaxationtimes[67].Multiplerelaxation timescanalsobeobservedwhenwaterislocatedinspatiallysep- aratedspotsinthe porousmatrix.Anexampleis amixedmicro- andmesoporousmatrixthathaswellseparatedmicropores.These micropores canbe filled before the mesopores,andsmallbottle- neckscan hindertheexchange ofwateramongdifferentporesat moderatefillingratios.However,thespatialseparationofwateris uncommon in highly hydrophilic porous solids, because thermo- dynamicpreferences dictatethe formationof a thick, continuous waterlayerthroughoutthenetwork[85].
Inthepresentstudy,relaxometrywasperformedwithhydrated silicaandsilica-gelatinaerogelsof differentwatercontent (i.e.at differentfilling ratios). InverseLaplace transformation ofprimary datashowsthe presence ofonly one waterrelaxationdomain in boththesilicaandthehybridsamplesuntila massratioof0.5g water/ g dryaerogel. At higherwatercontents, 2 relaxationdo- mainsarepresentinallsamples,asseeninFig8.(InverseLaplace plotsare shown Fig.S2 inthe Supporting Information). Inaccor- dancewiththe classicaltheory,we proposethat thefasterrelax- ationdomainrepresentsthehydrationsphereoftheaerogelback- bone,andtheslowerrelaxationdomainrepresentswatersituated fartherfromtheporewalls,e.g.inpuddlesforminginfocalpoints oftheaerogelnetwork.
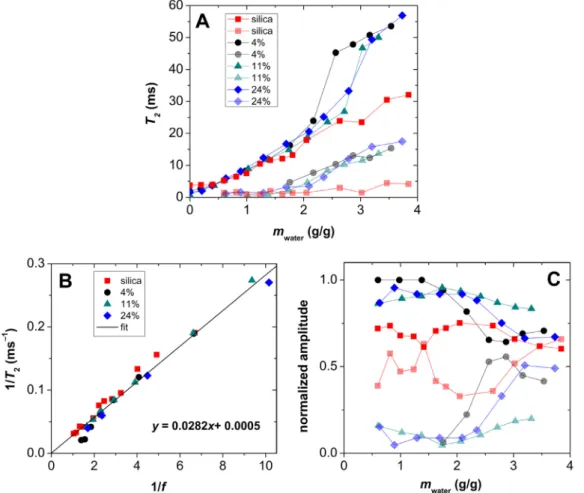
Forthequantitative evaluationoftherelaxometry dataofwet aerogels, first, the measured T2 relaxation times are plotted as function of water content (Fig. 8A), and second, the reciprocal value of T2 is plotted as function of the reciprocalvalue of fill- ing factor f(Fig. 8B). The total specific pore volumesof the wet aerogels, representing the unity filling factor (f = 1), were esti-
Fig. 8. NMR relaxometry of hydrated silica and silica-gelatin hybrid aerogels. Two relaxation domains are present for each aerogel at mass ratios higher than 0.5 g water / g aerogel. Panel A: measured T 2relaxation times as function of water content. Panel B: reciprocal T 2as function of reciprocal filling factor ( f ) for the fast relaxation domain.
Panel C: normalized amplitude of each relaxation domain (cf. panel A). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
matedfromNMRcryoporometrydataandarereferredtoasVpNMR
(Table 3). Therefore, the filling factor was calculated versus ca.
4.0cm3/gtotal specific porevolume (f= 1) forall aerogels. The massratiovaluesofaddedwaterwereconvertedtovolumebyus- ing1.00g/cm3 forthedensityofwater.
The 1/T2 vs. 1/f plots are meaningful only when constructed fortheuppermostwaterlayer,i.e.fortheslowestrelaxation(high T2) domain. As seen in Fig. 8B, the 1/T2 vs. 1/f plots are linear, andthus,followthetheoreticalequation derivedbySiminaetal.:
[63]
1 T2 =
ξ
VS0
1 fk+ 1
T2b
(6)
Here,
ξ
isthesurfacerelaxationstrength,Sisthe specificsur-faceareaandV0 isthetotalpore volume.Thevalue oftheinter- cept,i.e.thebulk waterrelaxationratewasfixed at1/T2b = 0.45 s–1.Thekexponentconveysinformationonthewettabilityofthe surface and the saturation mechanism of the pores. As demon- strated by the linearity of the plots in Fig. 8B, the value ofk is practically1forallhydratedaerogels.Overall,the1/T2vs.1/fplots suggestthat thetotal amountofsurfacewatercontributestothe fastrelaxationdomain.Thus, the fillingofthe pores ofthe aero- gelsisassumedtobeacontinuousprocess,whichbeginswiththe formationof a uniform,thick adsorption layerof waterthrough- out the solid network. At higher water contents (filling factors), puddlesappearinfocal pointsofthenetwork that forma quasi- bulkphase inside thepores. The k= 1 value suggeststhat there isastrong interactionbetweenthe homogeneoushydrationlayer andthepuddlesfillingtheporeinteriors.Importantly,thereareno
spatiallyseparated,preferredspots(e.g.insidemicropores)forwa- teraccumulation.Theseassumptionsarereasonable,sincebothsil- icaandsilica-gelatinareextremelyhydrophilicmatrices,[48]thus thermodynamic preferences dictate the continuous andhomoge- neousfillingofthepores.
Thenormalizedamplitudesofthefastandslowrelaxationdo- mains ofwater inthe aerogels(cf.Fig. 8A) aregiven asfunction ofwatercontentinFig.8C.Theamplitudeofadomainisgivenby thesize ofits inverseLaplace peak, andnormalizedamplitudeis calculated bydividing the amplitudewiththesum ofthe ampli- tudesofthedecays.AsseeninFig.8C,thenormalizedamplitudes ofthetwodomainsareinclosecorrelationwitheachotherinev- ery aerogel sample, especially at low water content (< 2.0 g/g).
Accordingearlierconsiderations, thiscan beattributedto amod- eratelyfast exchange betweenthe2 relaxationdomains ofwater inwetaerogels[62,66].Theamplitudesaremoreseparatedinthe case of the hydrated silica-gelatin aerogels, meaning that water exchange is slowerinthese materials than inthe hydrated silica aerogel,butitisstillnotintheslowexchangeregion.Thiscorrela- tionoftheamplitudesstrengthenstheabovedescribedmechanis- tictheory: thepores ofsilicaandsilica-gelatinaerogelsare filled bywaterinacontinuousprocesswhichformsinterconnectedlay- ers.Asthewatercontentincreases,largerpuddlesforminsidethe pores, andwaterinthesepuddlesexchange slowerwiththesur- facewaterlayer.
Oneremarkabledifferencecanbeobservedbetweenthehydra- tionmechanismofsilica andsilica-gelatinaerogels.The observed trend for the T2 values of hydrated silica aerogel is typical for solid,hydrophilicmesoporousmaterialsthatretaintheiropenpore
Fig. 9. Small angle neutron scattering (SANS) curves of silica and silica-gelatin hy- brid aerogels completely filled with D 2O (3.3 g liquid / g aerogel). Solid lines rep- resent data fitting to Beaucage model. Estimated structural parameters are given in Table 2 .
structures, characteristicattheirdrystate, evenwhencompletely filledwithwater[65].Incontrast,thereisanupwardbreakinthe trendoftheT2relaxationtimesofsilica-gelatinsamplesataround 2.0g/gwatercontent,asseeninFig.8A.Atwatercontentslower than thisthresholdvalue, thewetting processofsilica andsilica- gelatin is practically the same, andcan be attributed to the hy- drationofahydrophilicmesoporous solidnetwork.At highfilling factors,theT2valuessteeply increaseinsilica-gelatinsamplesfor both of the interchanging relaxationdomains. This indicates that water is no longer bound to well-defined solid surfaces or con- finedinwell-definedporesinthesesamples.Thus,itisreasonable to propose that athigher water content (>2.0g/g), silica-gelatin matricesreachacriticallevelofhydrationthatinducestheforma- tion ofhydrogel-like structures which isparallel to theextensive deformationofthehydratedaerogelbackbone.Thisprocessisalso well-expressed by the notedincrease oftheobserved singleself- diffusion coefficient of water by increasing water content in the caseofthe24wt.%gelatinaerogel(cf.Fig7).
3.2.6. SANSofhydratedaerogels
TheSANSscatteringcurvesobtainedforsilicaandsilica-gelatin aerogels saturated with 3.3 g/g D2O are shown in Fig. 9. These results representthe homogeneously hydrated (completely filled) statesofthe aerogels.No hydrationmicrodomainswere observed [86].
AllscatteringcurvescanbeadequatelyfittedwiththeBeaucage model.TheestimatedRgandpvaluesaregiveninTable2.Thegy- rationradiusslightlydecreasesuponthehydrationofeachaerogel, andpincreases.Themostobviousstructuralchanges,comparedto thedrystate, canbeobserved above11wt.%gelatin.The scatter- ing curve for thehydrated 24 wt.%gelatin hybridcontains infor- mation only in the Porod region meaning that the characteristic mesoporous network doesnot exist anymore in the sample. The value of the Porod exponent is highly above 4, which depicts a structurewheretheregionsofdifferentcontrastsareseparatedby agradientzone,insteadofasharpborder.Thisisin-linewiththe structuralinformationdeducedfromNMRdiffusiometryandrelax- ometry. The porenetwork ofthehybrid aerogelwiththe highest gelatincontentcollapsesinwaterandformsadensehydrogel-like matrix.
3.3. Summaryofstructuralcharacterization
Resultsfordryaerogelsby SANS,SEMandN2 porosimetryare ingoodagreementwitheachanother.All techniquessuggest that
thesolid frameworksofthehybrid aerogelsaresomewhat looser andlessbarred than thatofthe parentsilicaaerogel. Thecontri- bution ofthe macroporesto the porosity ishigher, andthe total mesoporevolume decreases withincreasinggelatincontent.Con- trast variation SANS experiments prove that silica-gelatin is hy- bridizedonthemolecularlevelinthesolidbackbonesoftheaero- gels.Thus,gelatindoesnotformanouter“layer” ontheinnerpore walls,orshowsanyspatialpreferenceinthematrix.
Nuclearmagneticresonancemethods(NMRcryoporometry,dif- fusiometry and relaxometry) and SANS showed that the well- definedmesoporousstructureofpristinesilicaaerogelispreserved whenthematerialisimmersedintowater.The pore-structuresof silica-gelatinhybrid aerogelssignificantly changeupon hydration.
Atlowgelatincontent(4–11wt.%),thestructureispermeableand open, yet not as open, as it is in its dry state. Hybrid aerogels withhigh gelatin content (18–24 wt.%) show hydrogel-like char- acteristics in water, because their open pore structure collapses dueto the extensivehydrationof their backbones. Acontinuous, soft matrix forms from these hybrids. Gelatin content also gov- ernsthedisintegrationofthemonolithicmatrices.Aerogelsofhigh gelatincontentyield smallermicroparticles inwater,because the secondary forcesbetweenthe proteinmolecules ofthe backbone are readilydisrupted by hydration, incontrast to thelimited hy- drolysisofthecovalentsilicanetwork.
3.4.Mechanismofdrugreleasefromaerogelcarriers
3.4.1. Structureofactiveingredientinaerogel
Silicaandsilica-gelatinaerogelswereimpregnatedwithibupro- fen andwith ketoprofen by using the well-establishedtechnique of adsorptiveprecipitation in supercritical CO2. The optimization ofthis techniquefor the impregnationof silica andsilica-gelatin aerogelmatriceshasbeencarriedoutbefore[24,40,41].
Theibuprofencontentoftheloadedaerogelsvariesbetween19 and24 wt.%, and the ketoprofen content varies between11 and 15 wt.% (Tables S1 and S2 in the Supporting Information). It is proved by XRD that theactive ingredients are amorphousin the loadedaerogels.Thespatialdistributionoftheactiveingredientis homogeneous onthe macroscopiclevel, asproved by FT-IRmea- surements.Thiswasensuredbytheconstantstirringandtheslow depressurizationoftheautoclaveduringadsorptiveprecipitation.
3.4.2. Designofdrugreleaseexperiments
Theloadingofthedrugswasrealizedbyusingdry,milledand sievedaerogelpowders. Theseloadedaerogel particleswere used inthereleasetests.Theloadedaerogelparticlesspontaneouslydis- integrateinaqueousmediatomicroparticles,eventuallygivingthe characteristicsizedistributions,asseeninFig.4.
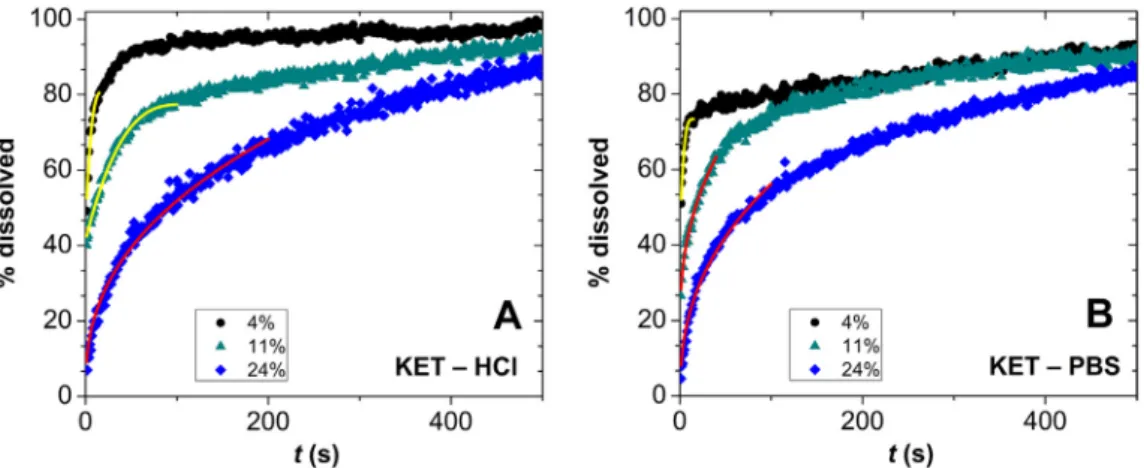
Thedetailedconditionsofthedrugreleaseexperimentsandthe solubilitiesoftheamorphousformsofIBUandKETaregiveninTa- blesS1andS2intheSupportingInformation.Sinkconditionswere fulfilledinthereleaseexperimentsofbothibuprofenandketopro- feninpH=7.4PBS,butnotinpH=2.0HClsolution.Atcomplete dissolutioninHCl, theconcentrationsofIBU andKETare equiva- lenttoca.80%and40% oftherespectivesolubilities. Representa- tivereleasecurvesareshowninFigs.10andS3forKETandinFig.
S4forIBU(intheSupportingInformation).
Itshouldbe notedfortheprofoundevaluationofthedrugre- leaseexperiments, that both IBU and KET are amorphous in the aerogel carriers. In general, the amorphous forms of drugs have higher solubilities and dissolve faster than the crystalline forms [87–91].Independentdrugreleaseexperimentswereperformedin pH=2.0HClsolutionbyusingdifferentweightsofloadedaerogel andequalvolumeofreleasemediumineachtest.Thisensuresthat thefinal drugconcentration is differentin each test. When drug releaseis given asthe percentage ofthe total amount of loaded