R O B E R T M . C H E W
Department of Biology, University of Southern California, Los Angeles, California
TABLE OF CONTENTS
I. Introduction 44 A. Outline of Water Balance Processes 45
B. The Effects of Domestication and Captivity 50 C. Water Exchanges and Habitat Distribution 51 II. Free Water Intake, by Drinking and in Food 53
A. Drinking of Free-Living Mammals 54
B. Free Water in Food 54 C. Drinking by Captive Mammals 59
D. Factors That Influence Drinking 65
E. Drinking Habits 72 F. Regulation of Drinking 72 III. Absorption of Water 76
A. Methods of Study 77 B. General Nature of Absorption 77
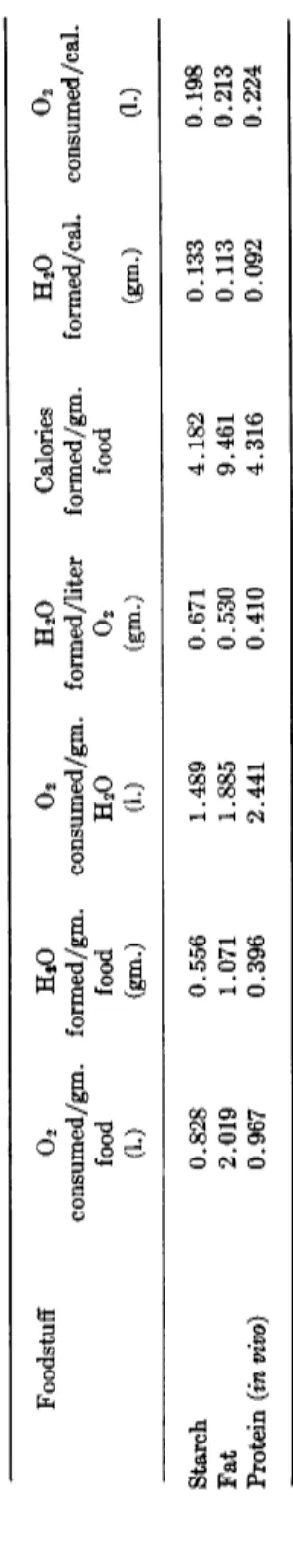
C. Endocrine Effects 78 IV. Oxidation Water 79
A. Role in Water Balance 79 B. Increased Metabolism as a Source of Water 81
V. Insensible Water Loss - - 81
A. Methods of Measurement 82 B. Data on Insensible Water Loss 83 C. Factors That Influence Insensible Water Loss 86
D. Nature of the Skin Barrier to Water 87
E. Respiratory Water Loss 89 VI. Homoiothermism and Water Metabolism 93
A. Physiological Indices of Evaporative Cooling 94 B. Water Balance and Sensible Heat Loss 95 C. Evaporative Water Loss and Thermal Stress 96 D. Dehydration, Drinking, and Thermoregulation 109
E. Renal Response to Heat Stress 110
F. Heat Storage 110 G. Behavior 111
1 Manuscript submitted April 1959 has been revised to include literature available through January 1, 1963, particularly that dealing with wild mammals.
Water Metabolism of Mammals
143
44 Robert Μ. Chew
I. Introduction2
Except for the work on kangaroo r a t s (Dipodomys) by the Schmidt- Nielsens and colleagues (1948, 1951), no thorough s t u d y has been made of the overall water metabolism of a n y truly wild m a m m a l . Certain aspects of water balance have been adequately studied in a b o u t fifteen other genera.
Two reviews have emphasized water balance of wild species, Fetcher (1939) for marine m a m m a l s and t h e Schmidt-Nielsens (1952) for desert m a m mals—both are groups t h a t have a t t r a c t e d special a t t e n t i o n because of their lack of n a t u r a l drinking water. I m p o r t a n t reviews of information on domestic m a m m a l s include those of Adolph (1933, 1943), Findlay(1950), and Leitch and Thomson (1944). Other reviews of water metabolism are predominantly or exclusively h u m a n and cellular in their orientation.
abbreviations Used: ACH-adrenal cortical hormones; ACTH-adrenocorticotropic hormone; ADH-antidiuretic hormone; B0-body weight; DOCA-deoxycorticosterone acetate; ECW-extracellular water; GFR-glomerular nitration rate; ICW-intracellular water; I.L.-insensible weight loss; I.W.- insensible water loss; I.W.S-I.W. through skin;
I.W.r-I.W. from respiratory surfaces; R.H.-relative humidity; RPF-renal plasma flow;
R.Q.-respiratory quotient; Ta-air temperature; Te x p-temperature of expired air; T in s p- temperature of inspired air; Tr-rectal temperature; T8-skin temperature; VPa-vapor pressure of air; VP8-vapor pressure of skin; Δ VP-skin to air vapor pressure gradient.
VII. Urine Water Loss 112 A. Urine Water Loss and Solute Excretion 113
B. Urine Volume in Relation to Other Water Exchanges 115
C. Theories of Kidney Function 116 D. Hormonal Factors in Urine Volume Regulation 121
VIII. Fecal Water Loss 127 IX. Body and Tissue Water Contents 129
A. Methods of Measurement 129 B. Total Water Content 130 C. Tissue Water Contents and Exchanges 132
X. Dehydration 134 A. Gross Effect on Water Exchanges 134
B. Changes in Water Compartments 136
C. Kidney Function 1-8 D. Metabolism and Respiratory Water Loss lc8
E. Limits of Tolerance 138 F. Recovery from Dehydration 140 XL Water Balance and Biological Processes 142
A. Development of Water Balance in Infant Mammals 142
B. Sexual Cycle, Pregnancy, and Lactation 145
C. Aging and Water Balance 148
D. Stress 148 Acknowledgments 149 References 149
The limited information on wild species obviously results from the fact t h a t docility and even cooperation of a trained animal subject is a necessity for much physiological work. In spite of the difficulties involved it is cer- tainly indicated t h a t more physiologists should extend their attention to the m a n y interesting problems presented by water metabolism of nonlab- oratory mammals and t h a t more mammalogists should broaden their interest in physiological aspects of mammals. The availability of ''tamed'' large mammals in zoos and marinelands offers a largely unexploited source of subjects for certain water balance studies.
This chapter will emphasize wild mammals as far as possible, b u t only by considering d a t a on domestic mammals is it possible to get an overall picture of water metabolism for the interpretation of d a t a on wild species and for the planning of future research. In certain aspects it is still necessary to refer to d a t a on h u m a n subjects.
A. Outline of Water Balance Processes
In Fig. 1 are diagrammed the component processes involved in the water balance of a generalized mammal. Regulatory mechanisms are indicated above each water exchange line; and stimuli on which regulation is based and limiting factors in exchange, below the lines.
Two of the exchanges can be considered to be balancing exchanges—the amount of water drunk versus the volume of "regulatory urine." Other exchanges are to a large degree coincident to the energy metabolism and
TOTAL BODY WATER DRUNK
IN FOOD
OXIDATION WATER
OBLIGATORY URINE
"REGULATORY"
URINE FECAL
WATER SKIN I.W.
^ / ^ o u i i^ R E S P I R A T O R Y I.W.
ANTING I.W.
SWEAT SALIVA FIG. 1. Water exchanges of a generalized mammal. Controlling mechanisms are given above the lines; stimuli bringing about exchanges and factors limiting the exchanges are given below the lines.
46 Robert Μ. Chew physical environment of the mammal. Although the amounts m a y change with extremes of hydration and dehydration of the body, this is more a consequence of altered hydration t h a n a regulation to meet it. When drink
ing water is not available, as is often the case, water balance is then largely a m a t t e r of reducing urine loss to within the "guaranteed i n t a k e " of water, i.e., t h a t provided coincident to feeding and metabolism. Unavoidable water loss in urine, feces and by evaporation m a y make balance impossible.
Thermal stress complicates balance, since heat regulatory mechanisms of sweating, panting, and salivation result in additional water losses.
Total body water is divisible into extracellular (ECW) and intracellular (ICW) compartments. Shifts of water into or out of the cells, without total volume change, can be as damaging as actual water loss or overhydration.
P a r t of the E C W is continuously circulating as blood plasma, and main
tenance of a minimum circulating volume is very critical to homeostasis.
Another p a r t of E C W can be considered to be in " s t o r a g e / ' particularly the water in connective tissues and t h a t cyclically accumulated in the female reproductive tract. The intestinal lumen is essentially continuous with E C W , though considerable volumes of water are shifted into and out of the lumen with the digestion of each meal.
The hypothalamus has the dominant neuroendocrine role in water regulation through its discrete drinking and feeding centers; its osmo
receptors and ADH-secreting nuclei; its temperature control centers which regulate respiratory volume, sweating, cutaneous blood flow, and salivation;
and its indirect influence on various endocrine glands.
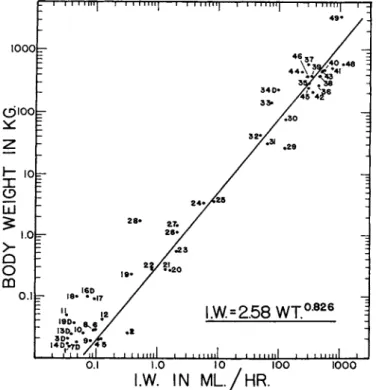
Figure 2 and Table I present the data of the few studies in which all pathways of water exchange have been measured or reliably estimated for normally hydrated animals under no thermal stress. The d a t a are p u t on a metabolic basis, units of water exchange per unit of oxidation water. A physiologically more significant comparison is for mammals forced to their minimum water expenditures by restriction of intake, as shown in Fig. 7.
However, Fig. 2 shows the levels of exchange t h a t occur with an unlimited water intake when presumably the animal is using as much water as needed to strike an optimum regulation of body water. Although comparisons are confounded by differences in experimental conditions, the following are indicated: (1) Only the desert rodent Dipodomys merriami fits its water expenditures easily within its "guaranteed water i n t a k e / ' (2) I n other mammals water expenditures are three to fourteen times the guaranteed intake. (3) Mammals of quite different sizes—rat, camel, steer—may have the same water turnover per unit of metabolism, although their absolute turnovers are very different. (4) There is no observable trend in proportions of different exchanges with size of the animal; as found by Adolph (1943), large mammals m a y have a proportionately greater fecal water loss, b u t this is not consistently true.
& 8 8 S j g t ! g g ~ q > K t f > r o - - i— ι — I — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι — ι —1 ι I I Γ"
"ΙΛΙ S V H D 3 H 3
3 S H 0 H XDOM
•ν,.".Λ·,.'.·.·..'..'.:;.:»;!;ν^.·.}·Ι
S M O O
NaoHiaoHS
A D AS833IS HE a smawvo
w UJ 0)
^ ζ υ
* 3 <l Ο χ
Ε
UJ 3Ο
Λ VOOHD
9 0 Q
Q ζ α:
ζ ο ^ χ ο
Ό Ό s n i o u o w
JLVH 3 1 Ι Η Μ
•w SAWoaodia
S 'IN Sn0SAW0d3d
' Ν Ί SN0SAIN0D3D
J I I — I — I — I — J — I — L J I I L J L J I I L
f»- ιΟ fO — φ
5
CNJ CM CM - IO tO — CD m ro —U 3 1 V M N 0 I 1 V Q I X 0 / 3 9 N V H 0 X 3 U 3 1 V M
FIG. 2. Total water exchanges of mammals allowed unlimited drinking water, compared on a metabolic basis. Absolute data, ex perimental conditions, and sources of data are given in Table 1.
TOTAL WATER BALANCE OF
TABLE I SEVERAL SPECIES OP MAMMALS WHEN DRINKING AD LIBITUM0 Water intake Water loss Species Body weight gm.
Water drunk ml.
Water in food ml.
Oxida tion water ml.
Urine water ml.
Fecal water ml.
I.W. ml.
Water Diet turnover ml.
Τ ± a R.H. Source Peromyscus leucopus noveboracensis 20.6 10.18 0.22 0.93 6.79 0.59 3.41 11.33 Purina chow 28 51 Chew (1951) P. maniculatus sonoriensis 19.1 2.65 0.56 1.17 2.16 WU 1.86 4.38 Purina chow 27 — R. L. French (1956), Chew (1955) Microtus o. ochrogaster 38.6 16.59 0.16 1.09 13.24 WU 4.42 17.84 Purina chow 28 51 Chew (1951) Blarina b. brevicauda 26.8 5.75 6.79 2.55 — — — 15.09 Horsemeat 19 55 Chew (1951) Dipodomys merriami 35 0.0 0.29 1.34 7.2 0.06 0.85 1.63 Barley 25 40 the Schmidt- Nielsens (1951) White rat 288 12.1 1.3 5.3 3.5 1.8 13.2 18.7 Mixed powdered 21 — Dicker and Nunn (1957) kg. I. I. I. I. I. I. I. Dog 17.2 0.79 — 0.25 0.33 0.08 0.62 1.04 Chow, milk — — Adolph (1943) Phoca vitulinac 18.2 — Herring 15 — Irving el al. (1935) Camelus dromedarius 243 3.18 0.34 0.54 0.73 1.22 2.12 4.06 Hay, dates 10 — B. Schmidt-Nielsen et al. (1956) 48 Robert M. Chew
Steers 391 8.53 1.23 1.65 3.80 2.52 6.20 11.63 Alfalfa 25 53 Mitchell and Hamilton (1936) Dry Shorthorn cows 632 32.2 1.23 1.26 13.6 13.5 6.64 34.7 Lucerne — — Balch et al. (1953) Work horses 611 15.7 61.0 6.9 21.8 37.3 24.5 83.6 Green feed — — Wittig (1938) Elephas maximus 3630 139 4.0 6.3 48.8 83.8 22.2 149.3 Hay 10 — Benedict (1936) a Absolute data for mammals compared on metabolic basis in Fig. 1. b WU, fecal water loss measured together with urine water. c No absolute data given.
50 Robert Μ. Chew Β. T h e Effects of Domestication and Captivity
Domestication and captivity m a y bring about genetic a n d / o r ecotypic changes in the processes involved in water balance of a mammal. T h e selection of domestic mammals for productivity in particular environments has undoubtedly resulted in genetic shiftings of the ranges of certain water exchanges. On the other hand, the altered environment of a captive animal m a y simply shift its physiological responses to a different p a r t of the existing genetic range t h a n would occur in a natural environment. Differ
ences between domesticated and wild breeds have been revealed in a few studies.
Richter and Mosier (1954) found t h a t about 3 0 % of wild Rattus nor- vegicus initially have unexpectedly variable and high water intakes in relation to their body size. Only after 30-50 days in captivity do their daily intakes become fairly constant a t quantities twice those of white rats.
Wild rats also drink more water in response to salt added to their food t h a n do white rats. Richter and Rice (1954) found t h a t fasting wild rats increase their activity five times more t h a n fasting domestic rats, and drink more water accordingly. These water intake differences m a y be due to the larger and more responsive adrenal cortex of the wild rat. High titers of endogenous A C H m a y cause greater thirst, as does injected D O C A in white rats. Steiniger (1952) also found larger fluid requirements for wild rats, b u t Henschel (1954) found no significant differences in water intake and urine, except t h a t wild rats more completely conserve chloride during deprivation of food and water.
Although wild house mice, Μ us musculus, have a higher rate of m e t a b olism t h a n domestic mice (Benedict and Lee, 1936), their evaporative water loss per milliliter O2 is lower (the Schmidt-Nielsens, 1950). The latter physiological difference is almost certainly genetic. Wolburg (1952) pro
poses t h a t evaporative heat dissipation is more strongly developed in wild mice. Thompson (1955) showed t h a t different domestic strains of mice respond differently to implantation of estradiol pellets; some increase their water turnover, while others show no change. Silverstein (1961) found significant differences in water intake, urine concentration, and urine specific gravity, among eight strains of white mice t h a t were allowed to drink ad libitum.
T h a t the laboratory environment is stressful to wild rodents is shown by t h e significant increase in adrenal weights of captive Gerbillus gerbillus (Burns, 1956); such stress can be expected to alter the water exchanges of captive wild mammals. Emotional stress increases drinking in white rats (Siegel and Siegel, 1949; Levine, 1958) and inhibits diuresis in rabbits (Brod and Sirota, 1949). White rats t h a t had not been handled when t h e y
were young drank less water in a later test period t h a n did individuals t h a t had been handled. However, when both groups were subjected to emotional stress, the nonhandled individuals showed a greater increase in drinking t h a n the handled ones (Levine, 1958). Elimination of sound stimuli can depress drinking in hooded rats (Weiss and Hurwitz, 1959).
Lindeborg (1952) found t h a t laboratory stock deer mice (Peromyscus maniculatus gracilis) drank significantly more t h a n individuals taken directly from t h e wild. Penned mule deer drink only about half the amount t a k e n b y wild mule deer (Nichol, 1938; Elder, 1954).
T h e opportunity which an animal has for exercise in captivity, and other conditions imposed by caging can significantly modify its water exchanges.
Citellus leucurus drink more water when they are kept in activity cages t h a n when t h e y are in terraria (Hudson, 1962). Peromyscus maniculatus spent t e n hours a day out of their nests when activity wheels were pro- vided, as compared to one and a half hours when there were no wheels (Kavanau, 1962); such increased activity would increase water losses.
Vampire b a t s (Desmodus rotundus) drink more blood when t h e y are caged singly t h a n when t h e y are in groups, probably because they are not crowded and jostled at t h e feeding bowl (Wimsatt and Guerriere, 1962).
Genetic difference in heat tolerance, involving evaporative cooling, are present in breeds of cattle and sheep. Indian breeds of cattle (Bos indicus) can more effectively control body temperature under thermal stress t h a n European breeds (Bos taurus) (Thompson et al., 1953; N a y , 1959; Allen, 1962). Among the European breeds, Jerseys show greater heat tolerance t h a n Shorthorns (Robinson and Klemm, 1953), possibly due to greater sweating. Merino sheep are superior in heat tolerance to Corriedales (Lee, 1950).
C. Water Exchanges and H a b i t a t Distribution
The availability of water in dry seasons and the physical factors causing water expenditure in specific habitats should act as factors in selection. I t is to be expected t h a t the water requirements and exchanges of each species of m a m m a l have evolved into a "fit" with its environment which precludes it from expanding its range to environments t h a t are more rigorous. Such fits have been demonstrated, and habitat limitations strongly indicated.
Genus Peromyscus has received special attention. Ross (1930) found a suggestive relationship between t h e presumed region of origin of a species and its drinking in captivity. Races of Peromyscus maniculatus (humid region) drank significantly more water t h a n races of P. eremicus (semiarid region), even when races from t h e same climatic region were compared.
However, comparisons of races within a species, even when extremes of climatic regions were involved, showed no significant differences.
52 Robert Μ. Chew Dice (1922) did not find differences between the water needs of captive P. maniculatus bairdi and P. leucopus ncveboracensis sufficient to be the basis for their habitat selection—dry grassland and adjacent deciduous forest, respectively. Desert subspecies of Peromyscus demonstrate better insulations at low air temperatures, and higher conductances at high temperatures, t h a n do nondesert subspecies. Desert deer mice, therefore, are able to maintain body temperature with less heat production and to lose heat more easily by radiation at high temperatures. Both these adjustments reduce the amount of water necessary for evaporative cooling ( M c N a b and Morrison, 1963).
Lindeborg (1952), in comparing the voluntary drinking of eleven races of five species of Peromyscus, found t h a t there were some significant differences in comparisons between races from different climatic regions, but not from different habitats in the same climate. The overall results of all work on Peromyscus support his conclusion t h a t the amounts of water consumed voluntarily in captivity are not very reliable for the estimation of water needs in nature. Lindeborg developed a more logical and effective basis for comparing races and species—their ability to maintain weight and survive on reduced rations of water. I n this comparison, the different races of Peromyscus, whether from the same macroclimate or not, fall neatly into order according to the judged xericity of their habitats.
Lee (1963), however, did not find such a correlation for wood rats.
Neotoma lepida from desert habitats in California drank more t h a n twice as much as N. lepida from semiarid coastal habitats and 5 0 % more than N.
fuscipes from chaparral habitats.
The high water requirement shown by the red-backed mouse (Cleth- rionomys gapperi maurus) in captivity is possibly a limiting factor in its habitat selection; C.g. maurus is found only in the wettest microhabitats (Odum, 1944). The fact t h a t the mountain beaver (Aplodontia ruja) cannot form a concentrated urine m a y be a factor limiting its distribution to moist coniferous forest (Pfeiffer et al., 1960).
I n a study of three small mammals t h a t occur together in Illinois, Chew (1951) found a general correlation between their water exchanges in cap
tivity and the "evaporative stress" of their microhabitats. The shrew, Blarina brevicauda brevicauda (subterranean, deciduous forest), has a water turnover twice t h a t of Peromyscus leucopus noveboracensis (surface, decid
uous forest). T h e prairie vole, Microtus ochrogaster ochrogaster has a very low evaporative loss—0.03 ml. per square centimeter per day at 28° versus 0.05 for P. I. noveboracensis and 0.12 for B. b. brevicauda. Probably Blarina could not maintain water balance outside its cool moist subterranean micro- habitat. The low evaporative loss of M. ochrogaster adapts it to conserva-
tion of water in dry seasons, but it is handicapped by insufficient evapor- ative cooling at high air temperatures. Mortality at 35-36° (Dice, 1922;
Chew, 1951) m a y limit its southward distribution.
H e r m a n n (1961) showed t h a t Microtus oeconomusy M. arvalis, and Cleth- rionomys rutilus, which enter the Russian steppe from the north, along water courses, have little resistance to desiccation and die after only a few days on air-dry foods. I n contrast, typical steppe rodents, Lagurus lagurus and Mus musculus, have a high resistance to desiccation.
Robinson and Morrison (1957) and Morrison (1962) have found, for the marsupials they have studied, t h a t thermoregulatory water exchanges are developed only in those species t h a t naturally need t h e m and t h a t have water available to effect them. Regulatory evaporative cooling is lacking in marsupial mice and rats and in burrowing bandicoots. Distribution of t h e macropods, which salivate copiously and lick themselves when over- heated, is probably limited by drinking water supply, as it is in the case of Setonyx brachyurus (Bartholomew, 1956). The American opossum, Didel- phis marsupialis, also uses saliva for cooling, and, according to Reynolds (1945), is distributed in close proximity to surface water.
Dipodomys panamintinus, a desert species, shows increased activity when under stress of water conservation, whereas D. morroensis, from a humid habitat, does not (Nichter, 1957). If such activity is an adaptation to produce a gain of oxidation water, it would not be expected in D. morroensis.
II. Free Water Intake, by Drinking and in Food
I t is relatively easy to measure the amount of drinking of mammals, and this has been done frequently. Less attention has been given to re- cording the times and quantities of individual drinks, which should be related to other activities; recording potometer devices are described by Gregersen (1932), Young and Richey (1952), and K a v e n a u (1962). Luick et al. (1959) describe a radioisotope method for estimating the water intake in the food of large grazing mammals. I n spite of the frequency with which drinking is measured, often little thought is given to its significance, relative to the intakes of free-living mammals, or to the factors t h a t influence its quantity in captivity. I t is necessary to recognize t h a t there is no single water requirement for a species or individual; t h e a m o u n t drunk depends upon factors such as: temperature, humidity, diet, frequency with which water is provided, conditions of caging, and stresses of the laboratory environment. T h e evaluation of published d a t a is very uncertain unless such experimental conditions are described.
54 Robert Μ. Chew A. Drinking of Free-Living Mammals
From field observations it is obvious t h a t m a n y individuals of m a n y species do not have surface water available for drinking and must get along without it. However, as for ground squirrels, Citellus, those individuals t h a t happen to live near water can be expected to use it (Grinnell and Dixon, 1918; Vorhies, 1945; Linsdale, 1946). Even Dipodomys drink from rain puddles (Tappe, 1941). Microtus californicus showed a highly significant selection of traps baited with a thimble of water, over traps t h a t were unbaited; water-baited traps were entered as frequently as those baited with rolled oats (Fisler, 1962). T h e fact t h a t the maximum activity of this rodent during the summer time occurs during the 4 hours after sunrise m a y be determined by t h e availability of dew at t h a t time of day (Pearson, 1960).
I t is necessary, however, to be careful in drawing conclusions from gross field observations. Although Taber (1945) observed armadillos (Dasypus novemcinctus) "miles from water," he found t h a t they became "ravenously t h i r s t y " after 5 days in pens without water. Field observation showed t h a t they frequent water holes, and, in time of drought, move to burrows near holes t h a t are still wet.
The group watering of gregarious mammals of deserts and grasslands is a characteristic life habit in these biomes. Probably m a n y of the m a m mals of more mesophytic biomes also drink, but observations are few:
Bailey (1923), Klugh (1927), and Beckwith (1957) for tree squirrels, Sciurus niger and Tamiasciurus hudsonicus] R a n d (1935) for Lemur catta.
The pouched rat (Beamys major), of the evergreen forests in Nyasaland, cannot be kept in captivity on a natural diet of seeds and fruits unless given drinking water, and apparently must drink in nature (Hanney and Morris, 1962).
Dew is often proposed as a source of drinking water, but its use is rarely observed. Linsdale (1946) records seeing Citellus beecheyi lick dew from leaves of plants around its burrow. Krumbiegel (1954) gives general refer
ences on use of dew and temporary catches of water in plant cups. Muir and Polder (1960) observed Myotis lucifugus drinking water t h a t had condensed on cave ceilings.
Snow is possibly the exclusive "drinking" water source of mountain sheep (Ovis canadensis) in the Gros Ventre mountains of Wyoming (McCann, 1956). Hares and tree squirrels eat snow and icicles (Klugh, 1927; Krumbiegel, 1954).
B. Free Water in Food
By force of circumstances, free water in their food is the exclusive or only dependable source of water for m a n y mammals. Because of the lack
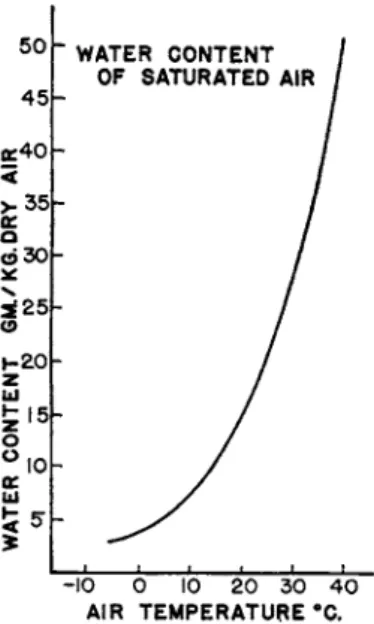
of succulent appearance of some foods, t h e quantity of water available m a y be underestimated. As shown b y t h e Schmidt-Nielsens (1951), t h e water content of "air-dry" barley a t 25° varies from approximately 3 . 5 % a t 1 0 % R . H . t o 1 8 % a t 7 6 % R . H . These relatively small amounts, plus water of oxidation, happen t o be sufficient for some mammals. These have so far proved t o be small rodents of arid a n d semiarid habitats t h a t feed pre- dominantly on seeds: Dipodomys spp., Perognathus spp., Microdipodops megacephalus (Hall and Linsdale, 1929), Jaculus loftusi and Gerbillus sp.
(Buxton, 1923), Meriones meriones (Howe and Jewell, 1959), Jaculus jaculus (Petter, 1961), and J . orientalis (Kirmiz, 1962), Different species of Dipodomys, Perognathus, and Meriones are not equal in their ability t o get along on air-dry food. Although P. fallax h a s been kept one t o eight years in good condition (Stephens, 1906; Howell and Gersh, 1935; Huey, 1959) and P . apache, seven m o n t h s (Vorhies, 1945), other species lose condition after a few weeks (Bailey, 1923; Benson, 1935). Dipodomys in general lose their good field condition after a short time in captivity without water. T h e small species such as D. merriami (40 gm.) a n d D. nitratoides (37 gm.) d o best (Culbertson, 1946); Z>. spectabilis (110-150 gm.) is "eager" for suc- culent food in captivity a n d eats some green food in nature (the Schmidt- Nielsens, 1951). Meriones libycus a n d M. crassus can be maintained in satisfactory condition a t least seven months on only air-dry barley, b u t M.
shawi, M. s. tristrami, M. vinogradovi, a n d M. persicus, which live in less arid natural habitats, must be given succulent foods (Petter, 1961). A t least some populations of Mus musculus can also do without water or suc- culent food (Hall, 1922; Chew, 1958); simple testing will probably show t h a t quite a few other seed feeders can do likewise.
Other mammals, however, need juicy food t o stay in water balance if they cannot drink. This occurs naturally with a flesh diet, b u t herbivores must choose t h e most succulent foods t o get an optimum water intake.
1. Herbivores
As Bailey (1923) h a s pointed out, foods of high water content are com- mon even in t h e desert. T h e food preferences of larger desert rodents clearly vary with a n d depend on t h e seasonal succulence of different plants (Vorhies a n d Taylor, 1933, 1940; Vorhies, 1945). T h e wood rat, Neotoma albigula, depends heavily on cactus, Opuntia ( 9 0 % water), which is about 4 4 % of its annual diet. Jack rabbits, Lepus californicus a n d L. alleni, eat seasonally succulent herbs a n d shrubs, using Opuntia when other succulent food is not present. Citellus tereticaudus shows no dependence on cactus, relying on green grasses a n d annuals, leaf buds and fresh fruits for its water. These rodents, a n d lagomorphs, show b u t slight use of d r y seeds a n d fruits. Citellus nelsoni does n o t use t h e unnutritious turpentine
56 Robert Μ. Chew weed and snakeweed until October when they are the only green plants
(Hawbecker, 1947); during summer and fall this species is primarily insectivorous. I n t h e desert-inhibiting C. leueurus, the extra water loss incident to its diurnal activity necessitates some water intake in succulent food. Individuals are usually in a semidehydrated condition when they are captured, and initially they have a high level of water consumption (Hud
son, 1962). I n favorable periods of the year C. leueurus can establish water credit (in terms of increased body weight), so they are not dependent on a continuously available water supply (Bartholomew and Hudson, 1959).
Psammomys obesus, a rodent of the Sahara desert, has a diet comprising almost exclusively parts of succulents of t h e family Chenopodiaceae, which are 78 to 8 9 % water. Captive animals often ignore other plant foods and dehydrate to the extent of 2 5 % of their body weight in two to three days
(Petter, 1961).
I n captivity, Microtus ochrogaster survives well on only fresh green grass (Dice, 1922), and Peromyscus spp. on grain, cactus, and lettuce (Ross, 1930). Captive jack rabbits live well on a mixture of d r y feed and green plants (Arnold, 1942). Wild rabbits (Oryctolagus cuniculus) t h a t are without drinking water gain weight through the spring, when the pastures are still succulent ( 6 8 - 7 7 % water in grasses), b u t lose weight when the pastures dry up in the summer (to as little as 7 - 1 0 % water in grasses) (Hayward, 1961).
N o t all green plants are equally satisfactory for water balance of Neotoma fuscipes, found in oak woodland and chaparral of California. I n particular, weight is lost rapidly on a diet of oak leaves and barley (Linsdale and Tevis, 1951). Significant differences exist between populations of Neotoma lepida in California. Individuals from coastal populations drink less water in captivity, and recover from dehydration more rapidly, t h a n do individ
uals from desert populations. Both forms can be maintained in the labora
tory on only pads of prickly pear (Opuntia) as a source of both food and water, although desert individuals do less well. Opuntia occurs naturally in t h e habitat of the coastal form b u t is not present in significant amounts in the habitat of the desert form. Desert N. lepida die after 11 days on air dry food, without water; when they are given only creosote bush (Larrea), the dominant plant in their habitat, they can survive 11 days, losing 2 2 % of their initial weight (Lee, 1963).
I n feeding trials, Voronov (1954) found t h a t water rats show definite preferences when they are offered hydrophytes; they choose plant foods having moisture contents from 67 to 8 7 % , and eat less of those plant parts having less t h a n 6 0 % water. Formozov and Kodachova (1961) found t h a t Marmota bobac chooses plants in which the water content is at least 5 0 - 5 5 % , whereas Citellus pygmaeus eats those in which there is as little as 3 0 % water.
T h e water of juicy plant foods is seasonally sufficient for certain large herbivores. Elder (1956) found minimum usage of artificial water holes by desert mule deer (Odocoileus hemionus) in Arizona after t h e winter rains.
Presumably sufficient water is derived from food to cover water expenditures in cool weather. Although water content of the deer's food plants did not decrease significantly, after April it was insufficient to cover the additional evaporative water losses incident to warmer weather. Taber and D a s m a n n (1958) present an analysis of moisture contents of chaparral plants browsed by deer; water content of the annual growth shows as much as a sevenfold annual variation. Leopold (1933) concluded t h a t succulent plant food provides t h e minimum moisture required by white-tailed deer ( 0 . vir- ginianus) in deciduous forests. T h e frequency of visits to waterholes by the desert bighorn sheep (Ovis canadensis nelsoni) varies seasonally; however, there is no evidence t h a t they can survive without some drinking water, even in t h e winter or a t higher elevations (Welles and Welles, 1961).
As shown by the observations of Gauthier-Pilters (1961) in the north- western Sahara, camels can get all t h e water they need in their food as long as air temperatures are only 30 to 35° or less. Camels pasturing on h a m a d a s m a y not come to drink from mid-September to April or M a y . Only when temperatures are above 40° do t h e camels make frequent trips to drink. Merino sheep, a breed t h a t presumably originated in a hot, arid environment, do not drink when grazing in winter in Australia (Macfarlane et al., 1958a). Since wild asses occur in parts of the central Gobi desert where "there is no water whatsoever" (Andrews, 1924) and Gazella arabica is found on waterless islands of the Red Sea (St. John, 1950), these herbi- vores m u s t maintain water balance on the water in their food. According to Krumbiegel (1954), porcupines, "Schliefer" (dachshund), and desert antelope go for a long time on only t h e moisture in their food, whereas
"Mendesantipole" (Addax) and " B a r e n p a v i a n e " (Papio tporcarius) per- manently satisfy their water needs with juicy plants. T h e koala abstains from drinking, and water given in captivity "is injurious." T h e kangaroo (Macropus robustus) is able to go without drinking when it has refuge from daytime heat in granite outcrops; there is one confirmed report of this species living in a desert area without access t o free water (Ealey, 1960).
T h e Barbary sheep (Ammotragus lervia) has often been described as going long periods without water in its natural habitats in Africa and as living even hundreds of miles from known water. This is supported by an observation on sheep introduced into New Mexico. Fifty animals kept in a three square mile game enclosure did not visit the only drinking water during 6 weeks of the hottest summer weather; pronghorn antelope, bison, elk, and deer did come t o drink (Ogren, 1962).
Macaca nemestrina drinks "surprisingly little" in captivity when fed moist foods (Krohn and Zuckerman, 1936), and food water is presumably
58 Robert Μ. Chew sufficient also for tree-dwelling m a m m a l s which rarely come down to t h e ground.
For other mammals, water in t h e food is an important complement t o drinking. The drinking of javelina (Pecari angulatus) varies inversely with the amount of prickly pear eaten (Sowls, 1958). I n the summer, mule deer drink 4 0 % less on a succulent diet t h a n on air-dry foods (Nichol, 1938).
Mule deer in paddocks on air-dry food drank three times as much as deer penned in spruce-fir habitat (Smith, 1954). When fed fresh grass on a year- round basis, sheep and goats drank on only 12.8% and 8.4% of days, re
spectively, whereas on a drier silage diet they drank on 5 2 . 7 % and 4 2 % of days (Asuncion, 1939). A complete substitution of food water for drinking occurred in grade cows switched from a silage-grain-hay diet to a fresh turnips-grain-hay diet; the total intake of water was actually higher on turnips (93.8% water) (Woodward and M c N u l t y , 1931). However, t h e drinking of captive Rattus norvegicus on a grain diet is practically unaltered after they are allowed large amounts of cabbage ( 9 0 % w a t e r ) ; each milli
liter of water from cabbage reduced drinking only 0.15 ml. (Chitty, 1954).
2. Carnivores
Carnivores, by the nature of their diet, get m u c h water with the flesh they eat. The grasshopper mouse, Onychomys torridus, can be kept at least 3 months in captivity on fresh mouse carcasses, b u t seeds are adequate only if drinking water is provided (Chew, 1958). Cats can live for long periods on flesh (Caldwell, 1931) a n d / o r fish t h a t is 6 7 - 7 3 % water (Prentiss et ai., 1959), but not on the same m e a t partly desiccated to a 5 9 - 6 3 % water content. During a severe drought in Africa the wild hunting dog Lycaon pictus was the only m a m m a l not perceptibly inconvenienced (Marais, 1915).
Domestic dogs can be maintained for weeks on haddock without drinking water (Danowski et aZ., 1944). T h e d a t a of Howard (1957) on the food intake of small carnivores allow t h e estimation of water intake in food as follows: Bassariscus astutus, 0.07 ml. per gram per d a y ; Felis domestica, 0.08 ml.; Mustela frenata, 0.23 ml. These intakes probably are sufficient for water balance since Fisher et al. (1938) kept cats on diets providing 0.09 ml. per gram per d a y without drinking. Blarina brevicauda drank consider
able water while on a horse meat diet, b u t probably h a d exaggerated water losses in captivity (Chew, 1951). Captive Spilogale putorius drink very little water when they are given a diet of live animals (Manano, 1961).
Marine mammals have received special attention since they have no fresh water for drinking. T h e fact t h a t no exceptional amounts of CI", M g+ +, or SO4 are found in t h e urine and feces of seals (Phoca vitulina) indicates t h a t they do not drink sea water. Calculations indicate t h a t P.
vitulina can maintain balance on t h e water it gets in a fresh herring diet, such
balance being aided by t h e fact t h a t there is no evaporative water loss through the skin (Irving et al., 1935; Smith, 1936). Similar calculations based on newer nutritional tables suggest t h a t seals could not maintain water balance on Atlantic herring, bluefish, halibut, Atlantic mackerel, or Pacific salmon, b u t could on Pacific herring, cod, flounder, haddock, and clams (Prentiss et al., 1959). These authors found considerable variability of composition of samples derived from the same kind of fish or meat, a fact indicating t h a t values taken from nutritional tables m a y be quite unreliable for such metabolic calculations.
Fetcher and Fetcher (1942) speculate t h a t whales which feed on inverte- brates (greater salt content t h a n teleost fishes) cannot maintain osmotic balance and excrete t h e urines of relatively low electrolyte concentration observed for them, unless they have a source of desalted water, possibly absorbed through their highly developed oral glands.
T h e drinking of sea water by marine mammals is certainly an open question. As demonstrated by Wolf et al. (1959) with cats, it is not necessary t h a t the concentrating ability of t h e kidneys exceed the concentration of sea water in order for a m a m m a l to derive benefit from drinking sea water.
If there is a sizable difference between the maximum osmolar concentration and the concurrent maximum salt concentration of the urine, then the salt-free nitrogen-obligated portion of t h e urine volume potentially con- stitutes the "osmotic space" into which some ingested salt can "escape"
and keep the total urine salt concentration below its ceiling.
C. Drinking by Captive M a m m a l s
For most free-living mammals t h e major water source is the fluid in their food, while for captive m a m m a l s it is drinking water. For newly captive m a m m a l s the satisfying of thirst by drinking must often be a unique experience. The relation of their water exchanges on this strange regimen of dry food and unlimited drinking water to their exchanges in nature is uncertain.
When water is present ad libitum, probably considerable water is used only to bring about moment-to-moment optimum balances in the body—
figuratively a "wasteful fine adjustment." On a restricted water intake, water balance is still maintained on a long-term basis, b u t possibly not as satisfactorily from moment to moment. T h e high voluntary intakes are reestablished almost exactly after a period of experimental restriction (Howell and Gersh, 1935; Chew, 1951). More work is needed on the minimum drinking requirements for normal maintenance and for reproduc- tion by m a m m a l s in captivity. I t would also be of considerable interest to determine water exchanges of captive m a m m a l s fed on their natural foods.
TABLE II DAILY WATER INTAKES OF MAMMALS Average Species Weight Water/day Diet temperature (°C.) Source 1. Blarina b. brevicauda gm. 25.8 ml. 12.5 Horsemeat 19 Chew (1951) 2. Peromyscus leucopus noveboracensis 22.9 2.6 — — Odum (1944) 3. P. leucopus noveboracensis 19.1 1.7 Whole wheat 21 Dice (1922) 4. P. leucopus noveboracensis 20.7 5.4 Purina dog chow 18 Chew (1951) 5. P. leucopus noveboracensis 21.6 2.5 "Air-dry food" 20-25 Lindeborg (1952) 6. P. leucopus torniUo 29.4 1.9 "Air-dry food" 20-25 Lindeborg (1952) 7. P. maniculatus bairdi " 18.6 3.0 "Air-dry food" 20-25 Lindeborg (1952) 8. P. maniculatus bairdi 13.8 1.7 Whole wheat 21 Dice (1922) 9. P. maniculatus blandus 24.0 2.7 "Air-dry food" 20-25 Lindeborg (1952) 10. P. maniculatus nebrascensis 19.7 1.9 "Air-dry food" 20-25 Lindeborg (1952) 11. P. maniculatus gracilis 21.5 2.6 "Air-dry food" 20-25 Lindeborg (1952) 12. P. maniculatus sonoriensis 19.1 2.7 Purina dog chow 25 R. L. French (1956) 13. P. maniculatus nubiterrae 17.3 1.8 — — Odum (1944) 14. P. frwei irwei 32.4 2.8 "Air-dry food" 20-25 Lindeborg (1952) 15. P. eremicus eremicus 21.3 2.1 "Air-dry food" 20-25 Lindeborg (1922) 16. Clethrionomys gapperi maurus 27.9 25.0 — — Odum (1944) 17. Microtus p. pennsylvanicus 28.3 6.0 Oats-dry grass 21 Dice (1922) 18. Μ. p. pennsylvanicus 34.7 7.2 "Air-dry food" 20-25 Lindeborg (1952) 19. Μ. ο. ochrogaster 42.6 15.8 Purina dog chow 28 Chew (1951) 20. Pitymys pinetorum 18.2 1.8 — — Odum (1944) 21. Mws musculus albino 23.6 4.1 Powdered stock ration Lab Bing and Mendel (1931) 22. Μ. musculus albino 22.4 2.7 Seed ration Lab Bing and Mendel (1931) 23. M. musculus albino 22.4 4.4 Powdered stock ration Lab Bing and Mendel (1931) 60 Robert M. Chew
24. Μ. musculus albino 31.7 25. Μ. musculus albino 35.1 26. Dipodomys panamintinus 79.0 27. D. morroensis 68.0 28. D. ordii 44.0 29. D. o. columbianus 44.0 30. Rattus norvegicus 230 30a. Citellus leucurus 85.0 31. R. norvegicus 407 32. R. norvegicus 75 33. β. norvegicus 394 34. β. norvegicus 130 35. β. norvegicus albino 126 36. β. norvegicus 241 37. β. norvegicus albino 241 38. β. norvegicus albino 200 39. β. norvegicus albino 288 40. β. norvegicus albino 225 41. β. norvegicus albino 128 42. Neotoma pennsylvanica 200 10.5 Purina chow 22 Chew and Hinegardner (1957) 12.0 Purina chow 22 Chew and Hinegardner (1957) 16.0 Pearled barley 25 Nichter (1957) 14.8 Pearled barley 25 Nichter (1957) 1.2 Pearled barley — Lindeborg (1958) 6.0 Pearled barley — Howell and Gersh (1935) 24.6 Whole wheat Lab Chitty (1954) 10.2 Shelled sunflower seeds 19-25 Bartholomew and Hudson (1959) 29.6 Whole wheat Lab Chitty (1954) 10.7 Whole wheat Outdoor Chitty (1954) 26.8 Whole wheat Outdoor Chitty (1954) 32.2 Mixed diet Lab Richter and Rice (1954) 23.9 Mixed diet Lab Richter and Rice (1954) 18.6 Grain and mixed moist Lab Henschel (1954) food 25.4 Grain and mixed moist Lab Henschel (1954) food 29.2 Dry milk 22-28 Adolph (1943) 34.8 Dry mixed diet 21 Dicker and Nunn (1957) 35.4 Dry mixed diet Lab Richter (1938) 19.6 Mixed diet Lab Crampton and Lloyd (1954) 113 Mixed diet — Patterson (1933)
TABLE II—Continued Average Species Weight Water/day Diet temperature (°C.) Source kg. liter 43. Cat 4.49 0.32 — Lab Richter (1938) 44. Macaca nemestrina 5.45 0.39 Variety, succulent Lab Krohn and Zuckerman (1936) 45. Dog 10.7 0.60 Variety, succulent Lab Richter (1938) 46. Dog 16.7 1.07 Fox chow, milk Lab Adolph (1939) 47. Dog 10.6 0.75 Fox chow, milk Lab Kleitman (1927) 48. Papio ursinus 21.1 1.31 Variety, succulent Lab Gillman and Gilbert (1956b) 49. Pecari angulatus 23.6 2.2 Prickly pear — Sowls (1958) 50. Odocoileus h. hemionus 24.4 1.52 Bitterbrush 4 Bissell et al. (1955) 51. O.h. hemionus 25.6 1.04 Sagebrush 4 Bissell et al. (1955) 52. 0. h. hemionus 19.1 1.90 Alfalfa 7 Bissell et al. (1955) 53. 0. hemionus columbianus 27.2 2.90 Live oak-alfalfa 19 Bissell and Weir (1957) 54. 0. hemionus columbianus 24.4 3.56 Alfalfa 19 Bissell and Weir (1957) 55. 0. hemionus columbianus 24.6 3.33 Chamise-alfalfa 20 Bissell and Weir (1957) 56. 0. hemionus macrolis 45.4 1.5 Hay Jan. Nichol (1938) 57. 0. hemionus macrolis 45.5 4.18 Hay July Nichol (1938) 58. Sheep, Merino 27.7 2.10 Wheat-meal-lucerne 23-28 Riek et al. (1950) 59. Sheep, Corriedale 52.7 2.06 Wheat-meat-lucerne 23-28 Riek et al. (1950) 60. Sheep, Merino 33.1 0.24 Brush hay-corn max. 22 Macfarlane et al. (1958a) 61. Sheep, Merino 26.7 1.40 Brush hay-corn 31 Macfarlane et al. (1958a) 62. Pigs, growing 45.5 8.38 Brush hay-corn 21 Bond et al. (1952) 63. Pigs, fattening 149.9 8.7 Brush hay-corn 21 Bond et al. (1952) 64. Camelus dromedarius 243 3.18 Hay-dates — B. Schmidt-Nielsen et al. (1957) 62 Robert M. Chew
65 Zebu cattle, d"d\ dry 9 9 145 11.19 Fresh grass-cassava-/cot-22 Rollinson tt al. (1955) tonseed 66. Zebu cattle, o" d\ dry 9 9 157 12.68 Fresh grass-cassava 22 Rollinson et al. (1955) cottonseed 67. Holstein, Jersey lactating 9 9 427 17.92° Hay Woodward and McNulty (1931) 68. Shorthorn, dry 9 9 632 32.24 Hay 20 Balch et al. (1953) 69. Steers 391 8.53 Corn-alfalfa 24 Mitchell and Hamilton (1936) 70. Work horse 611 76.0 Corn-alfalfa "warm Wittig (1938) weather" 71. Elephas maximus 3630 139.0 Hay 10 Benedict (1936) Addendum. Data not plotted in Figure 3 gm. ml. Desmodus rotundus
—
15.5 Defibrinated blood—
Wimsatt and Guerriere (1962) White mice (8 strains) 24-34 4.3-10.0 Purina laboratory chow—
Silverstein (1961) Meriones unguiculatus 94 3.7 Rolled oats 20-25 Winkelmann and Getz (1962) Citellus leucurus 92.5 13.4 Rolled oats 22-25 Hudson (1962) Neotoma lepida (desert) 110 36.6 Rolled oats 22-25 Lee (1963) N. lepida (coastal) 139 18.1 Rolled oats 22-25 Lee (1963) N. fuscipes 187 45.7 Rolled oats 22-25 Lee (1963) Rabbits, Dutch breed 2140 201 Rockland pellets 20-23 Cizek (1961) kg. I. MacacamuMta — 0.6-2.3 — — Feldmahn et al. (1960) Kumauni bullocks 340 8.9 Roughage and concentrate 21 Negi and Mullick (1960) ° Corrected for water in milk.64 Robert Μ. Chew
LITERS DRUNK PER DAY
FIG. 3. Relationship between body weight and amount of water drunk for various mammals ranging in weight from 13.8 gm. [deer mouse (Peromyscus)] to 3630 kg. (ele
phant). Numbers refer to listing of data in Table I I . Unnumbered points in lower left of figure are for Peromyscus subspp., numbers 2-15 in Table I I .
Significant differences in ad libitum water intake can exist between populations of the same species as shown by Lee (1963) for Neotoma lepida, and by Silverstein (1961) for eight pure strains of white mice. Most of the eight strains maintained a level of drinking t h a t was in keeping with a condition of water scarcity, as judged from their urine concentrations, although unlimited drinking water was available.
Figure 3 and Table I I give a summary of d a t a on drinking intakes of various-sized mammals in captivity. The only unifying factor in these d a t a is t h a t all values are for comfortable temperatures, and, except where indicated, the diet was air-dry food.
T h e amount of water drunk by normally hydrated animals is correlated with body weight; liters drunk per day = 0.098 wt. (kg.)0-9 0 3. This is very close to the relationship found by Adolph (1943), on the basis of m a n y fewer species, t h a t total water intake is proportional to B0 0-8 8.
A relationship of drinking t o metabolism was first proposed b y Richter and Brailey (1929), who found t h a t rats varying in weight from 50 to 325 gm. all d r a n k t h e same a m o u n t of water per square meter of body surface area. Richter (1938) proposed a constant intake of about 1100 ml. per square meter for mammals ranging in size from rat to man. The d a t a of Siegel and Stuckey (1947) indicate t h a t t h e water i n t a k e : b o d y weight relationship is secondary to a weight:food (energy) and food:water re- lationship. R a t s showed a significant correlation of water to surface area when permitted to eat, b u t not when fasted.
D . Factors T h a t Influence Drinking
These factors can be classified according t o whether they involve: (1) Increase in concentration of body fluids due t o loss of water. This can be counteracted only by drinking, and t h e quantity d r u n k will vary directly with the loss and inversely with other intakes. (2) Increase in concentration due to shifts of water a n d / o r electrolyte within the body, such as shift of water from E C W into digestive t r a c t in digestive juices. Such changes m a y be corrected relatively soon by reverse shifts within t h e body, b u t they can also be immediately corrected by drinking. Such drinking represents a
"surplus fine adjustment" not essential for long-term water balance. (3) N o concentration change, b u t some stimulus such as taste or emotional stress, acting via the nervous system. I t m a y be impossible even partly t o dissoci- ate the effects of these three kinds of factors on t h e total water intake of a particular animal.
1. Temperature and Humidity
Several studies show seasonal differences in drinking referable to weather.
Mule deer in pens in Arizona drank two and a half times more in July t h a n in J a n u a r y (Nichol, 1938). Merino sheep in Australia, kept in unshaded yards, d r a n k twelve times more in summer t h a n in winter (Macfarlane et al.}
1956). Citellus nelsoni in captivity drank "more in warm weather t h a n cold" (Hawbecker, 1947). T h e frequency and volume of drinking by camels is clearly related to seasonal climatic changes, as these determine both t h e need of the camels for evaporative cooling and the water content of their forage (Gauthier-Pilters, 1961).
T h e drinking of dairy cows (Winchester and Morris, 1956) and white rats (Mefferd et aZ., 1958) is minimal between 10 and 20° and increases rapidly with higher temperatures. Greater drinking at lower temperatures is associated with increased food intake, whereas t h e decrease t h a t occurs at t h e highest temperatures is p a r t l y due t o a depression of feeding. T h e
66 Robert Μ. Chew effects of feeding and of temperature can be dissociated b y : (1) assumption of the minimum observed water:food ratio as the obligatory relationship and subtraction of this amount from the total intakes at all temperatures;
(2) as suggested by Mefferd et al. (1958), taking the water intake of a fasting animal as an index of water regulation related to temperature. When the above d a t a for drinking of cows and rats are so corrected for feeding effect, the amount drunk then shows a consistent increase from the lowest to highest temperature.
Effects of humidity are obvious only at higher air temperatures. White rats at 29° drink significantly more at 1 0 - 2 0 % R . H . t h a n at 8 0 - 1 0 0 %
(Kligler et al., 1945), and the same is true for cows at 18.5° (Ragsdale et αι., 1953) and for bullocks at 27° (Negi and Mullick, 1960).
Low humidities can result in increased water intake, both by their effect in increasing evaporative water loss and increasing feeding. For example, Chernomordikov (1962) found t h a t both Rattus norvegicus and R. rattus, kept at 15°-18°, eat progressively more as the relative humidity is decreased from 8 0 % to 5 0 % .
2. Feeding
The feeding-drinking relationship involves three things: quantity of food eaten, the organic composition of the food, and solute content of the diet.
a. Quantity. When unlimited water is available, there is a close relation
ship between the amount of a particular diet eaten and the amount of water drunk. I n dogs the same food:water ratio is consistent over three to four years (Cizek, 1959). I n growing rabbits there is an essentially linear relationship between water and food intake, and in adult rabbits the ratio remains constant over periods of 6 months or more (Cizek, 1961). If the food intake is experimentally increased, water intake is usually increased in proportion, as when hyperphagia is induced b y : brain lesions in r a t s
(Strominger, 1947; Bruce and Kennedy, 1951); vagotomy in dogs (Towbin, 1955); exogenous thyroxine in rats (Richter, 1933); and dilution of food with sawdust in rabbits (Abgarowicz, 1948). The same water:food ratio holds for domestic or wild Rattus norvegicus of different weights (Richter and Brailey, 1929; Siegel and Stuckey, 1947; Chitty, 1954).
Zebu cattle (Bos indicus) have a lower water:food ratio t h a n European breeds (B. taurus) on the same diet. The water:food ratio of cattle fed on submaintenance, maintenance, and liberal rations did not vary significantly from 2.96 ml. per gram (Winchester and Morris, 1956). However, Riek et al.
(1950) found t h a t the ratio varied in sheep from 0.114 ml. per gram on a low nutritional plane to 0.053 on a high plane.
b. Organic composition. I n tests of different diet combinations on rats, Schreiber and Elvehjem (1955) and Bruce and Kennedy (1951) found t h a t drinking increases with protein content, and on diets of t h e same protein content the least drinking occurs with high carbohydrate and low fat.
Greater drinking on high-protein diets has also been found for rats by Adolph (1947b), Aaes-Jorgensen and D a m (1954), and Sarett and Snipper (1956) and for cattle by Winchester and Morris (1956). I n Peromyscus maniculatus drinking is initially depressed after switching from Purina Chow ( 2 3 % protein) to crude barley ( 8 % protein) and initially increased after switching to soybean meal ( 4 4 % protein); b u t in both instances drinking intake returned to the base value established on Chow, after 5 weeks, indicating a physiological adjustment to the new protein level (Williams, 1959). Added protein increases obligatory urine volume, whereas carbohydrate "spares drinking" because it provides the most oxidation water per calorie.
Although water drunk per gram of food varies considerably with diet composition, water per calorie shows considerable constancy (Adolph, 1947b; Bruce and Kennedy, 1951; Schreiber and Elvehjem, 1955). Bing and Mendel (1931) did find the same milliliter: gram ratio for white mice on different diets, and from this concluded t h a t drinking is for lubrication.
However, it is more probable t h a t drinking is predominantly related to metabolism: i.e., renal "demands for excretion," oxidation water, respira- tory water loss. Cizek (1961J concludes t h a t the relationship of water intake to food intake is best explained in terms of the osmotic effect of the food, as it displaces extracellular water into the lumen of the digestive tract.
Only when drinking is severely restricted are white mice unable to lubircate food adequately for swallowing (Chew and Hinegardner, 1957).
When food is diluted with water, large water surpluses will be taken in obtaining the same caloric intake. R a t s maintain caloric intake on diets as dilute as 2 . 5 % solids (Adolph, 1947b; Bruce and Kennedy, 1951). There is no evidence t h a t animals will t a k e a caloric surplus in order to obtain more water; however, they m a y select the more succulent foods.
c. Solute content. When salts (NaCl, KC1, K H C 03) are added to a r a t ' s solid diet, its drinking is increased 50-60 ml. per gram solute (Gamble et al., 1929; Richter and Mosier, 1954). Urea alone causes drinking increments of 15-20 ml. per gram, but when urea and N a C l are given together, drinking is no greater t h a n if only N a C l had been given (Gamble et al., 1929; Adolph et al., 1954). Hereford heifers d r a n k an additional 230-440 ml. of water for each gram of salt ingested, when 1 % and 2 % salt was added to their diet of chopped alfalfa hay (Weeth et al., 1960).
Such d a t a have been interpreted as supporting the idea t h a t drinking is determined by "renal d e m a n d s . " However, much of the drinking incre-