Pentahalides of the Transition Metals
A . D . B E V E R I D G E a n d H . C. C L A R K
Department of Chemistry, University of Western Ontario, Londoji, Ontario, Canada
1. I n t r o d u c t i o n . . 179
2. P e n t a h a l i d e s . .
A . P h y s i c a l P r o p e r t i e s a n d S t r u c t u r e B . P r e p a r a t i o n o f t h e P e n t a h a l i d e s C . C h e m i c a l P r o p e r t i e s
3. M i x e d P e n t a h a l i d e s . . R e f e r e n c e s
1 8 0 1 8 0 1 9 0 195 2 1 8 2 1 9
1 , Introduction
T h e p e n t a h a h d e s a r e a m o n g t h e m o s t interesting halogen-containing derivatives, from t h e p o i n t of view of b o t h t h e i r chemical b e h a v i o u r a n d t h e i r s t r u c t u r a l characteristics. W e shall b e concerned here firstly w i t h t h e p e n t a h a l i d e s of t h e t r a n s i t i o n m e t a l s , t h u s covering t h e ele
m e n t s i n periodic g r o u p s V Β t o V I I I inclusive. I n a d d i t i o n , some of t h e halide complexes containing t h e s e t r a n s i t i o n m e t a l s i n t h e + 5 oxi
d a t i o n s t a t e will also b e reviewed. These topics i n themselves c o n s t i t u t e such a large field of reference t h a t oxyhalides will n o t b e discussed specifically, a l t h o u g h t h e m a j o r i t y will p r o b a b l y b e a t least m e n t i o n e d in passing. N o r is a n y discussion included of t h e p e n t a h a l i d e s or p e n t a v a l e n t complexes of t h e actinides. R a t h e r , i t is h o p e d t o correlate t h e physical a n d chemical properties w i t h t h e available s t r u c t u r a l infor
m a t i o n for t h i s m o r e closely defined series of halogen c o m p o u n d s , t a k i n g i n t o a c c o u n t reviews of p e n t a h a l i d e s of t h e groups V A e l e m e n t s ( n i t r o g e n - b i s m u t h ) .
T h e t r a n s i t i o n m e t a l s w h i c h form p e n t a h a l i d e s a n d p e n t a v a l e n t halide complexes include v a n a d i u m , n i o b i u m , t a n t a l u m , c h r o m i u m , m o l y b d e n u m , t u n g s t e n , t e c h n e t i u m , r h e n i u m , r u t h e n i u m , r h o d i u m , o s m i u m , iridium, a n d p l a t i n u m . I n e v i t a b l y , for such a high o x i d a t i o n s t a t e , fluorides a r e t h e m o s t a b u n d a n t , a l t h o u g h certain of these m e t a l s form all four p e n t a h a l i d e s . Comparisons will b e m a d e over t h e entire r a n g e of t h e s e c o m p o u n d s , commencing w i t h a discussion of t h e physical b e h a v i o u r of t h e p e n t a h a l i d e s .
1 7 9
180 Α. D . BEVERIDGE AND H. C. CLARK
2. Pentahalides A, Physical properties and structure
T h e available values of physical constants for t h e pentahalides are presented i n Tables I - V . Measurements of some of these properties were m a d e m a n y years ago, a n d frequently these older values are unreliable because of t h e difficulties encountered i n obtaining t h e pentahalides i n high p u r i t y . F o r example, i t is claimed (Colton a n d T o m k i n s , 1965) t h a t pure m o l y b d e n u m pentachloride was obtained only i n 1965, a n d t h a t after the r e m o v a l of trace amounts of m o l y b d e n u m oxide tetrachloride, m a r k e d l y higher values were recorded for t h e magnetic susceptibility of t h e pentachloride. Similar i m p r o v e m e n t s h a v e been recorded i n t h e physical constants of other pentahalides, a n d t h e values quoted i n t h e following tables are based on a careful assessment of the p u r i t y of t h e samples used.
(i) Pentafluorides of Vanadium, Niobium, and Tantalum
These pentafluorides are all w h i t e solids whose m e l t i n g points v a r y f r o m 19-5° for VFg, t o 95-1° for TaF5. T h e i r simple physical charac
teristics provide strong evidence for t h e existence of polymeric units i n t h e l i q u i d state. T h e T r o u t o n constants are higher t h a n the n o r m a l '*non-associated" value of a p p r o x i m a t e l y 2 1 , a n d t h e viscosities are also v e r y h i g h , being exceeded a m o n g inorganic fluorides only b y t h a t o f a n t i m o n y pentafluoride (460 centipoise a t 25°) ( W o o l f a n d Greenwood, 1950). Perhaps surprisingly, t h e surface tension of v a n a d i u m penta
fluoride is low i n comparison w i t h other associated fluorides although this q u a n t i t y is a m u c h less reliable indication of molecular association.
I n general, t h e properties indicate t h a t v a n a d i u m pentafluoride is more highly associated t h a n t h e n i o b i u m or t a n t a l u m pentafluorides, a n d moreover, t h a t t h e v a n a d i u m compound undergoes a greater degree of self-ionization as shown b y its higher specific conductance. E x p e r i m e n t a l values for t h e heats of f o r m a t i o n of t h e v a n a d i u m a n d n i o b i u m penta
fluorides are given i n T a b l e I , a n d t h e value of —432 kcal mole-^ for N b F g can be compared w i t h values of —370 (Amosov, 1963) a n d —466 kcal mole-^ (Cavell a n d Clark, 1965) w h i c h have been calculated b y empirical methods. Similar calculations (Amosov, 1963) give AH^^j.^, for T a F g as —380 k c a l mole-^. T h e h e a t of hydrolysis of v a n a d i u m pentafluoride is given as ΑΗγ^γ^ = —141 ± 4 k c a l mole-^ (Cavell a n d Clark, 1963a).
O t h e r t h a n t h e above conclusions based on their physical properties, there is no i n f o r m a t i o n available concerning t h e structures of these pentafluorides i n t h e l i q u i d state. V a n a d i u m pentafluoride is mono- meric i n t h e vapour, as shown b y its vapour density, b u t t h e correspond
ing measurements h a v e n o t been m a d e on t h e other pentafluorides.
PENTAHALIDES OF THE TRANSITION METALS 1 8 1
V F , N b F , T a F s
Melting point (°C) 19.5α,6 80-0^'^ 9 5 - l e 96-97^
Boiling point (°C) 48·3« 47·9& 234-9^ 229-2C
Molar v o l u m e (cm^ mole~^) 5 8 - 3 3 / 6 9 - 7 1 / 7 1 - 1 0 /
D e n s i t y (g cm-^) 2-502 (at 20°)^ 2-6955 (at 80°)^ 3-8800 (at 95°)'^
Viscosity (cpoise) 124 (25°)^^ 91-41 (80°)/ 70-31 (95-1°)/
Specific c o n d u c t a n c e
70-31 (95-1°)/
( X 105 o h m - I c m - I ) 24-3 (25°)« 1-63 (80°)/ 1-56 (95-1°)/
Surface tension (dyne cm-^) 18-2 (25°)fl' —
Vapour d e n s i t y Δίί°formation (kcal mole-^) - 3 5 21 4 6 - Ρ ±éh -432* —
—
—Aiîvap (kcal mole-^) 10-64« lO-eO'' 12-9^ 13-0^
AxS'vap (Trouton constant) 33·1« 25-4C 25-9C
AHfusion (kcal mole-^) 11·94« 4-2^· 8-6^ —
2-92^
A/Sfuslon (e.u.) — 1-62^ —
« Clark a n d E m e l e u s (1957). Cavell a n d Clark (1963a).
b Trevorrow et al. (1957). ^ Cavell and Clark (1963b).
c Fairbrother a n d F r i t h (1951). « Myers a n d B r a d y (1960).
^ J u n k i n s et al. (1952). ί Brewer (1951).
β Muetterties a n d Castle (1961). ^ B r a d y et al. (1960).
/ F a i r b r o t h e r et al. (1965a).
T h e infrared s p e c t r u m of t h e v a p o u r of VF5 h a s been i n t e r p r e t e d (Cavell a n d Clark, 1 9 6 4 ) i n t e r m s of a t r i g o n a l b i p y r a m i d a l m o n o m e r i c s t r u c t u r e , a l t h o u g h t h e associated R a m a n s p e c t r u m is necessary for complete conflrmation. E v e n ^^F n u c l e a r m a g n e t i c resonance studies of p u r e v a n a d i u m pentafluoride a n d its solutions, h a v e b e e n unsuccess
ful in o b t a i n i n g information a b o u t t h e degree or m o d e of association.
T h e reasonable suggestion h a s been m a d e ( F a i r b r o t h e r et al., 1 9 5 4 ; Clark a n d E m e l e u s , 1 9 5 7 ) t h a t , in t h e liquid s t a t e , polymeric species formed t h r o u g h fluorine-bridging are i m p o r t a n t , w i t h some small degree of self-ionization t o a c c o u n t for t h e electrical c o n d u c t a n c e .
(MF,)„ ^ ^ M F , + + ^ M F e -
I n t h e solid s t a t e , complete c r y s t a l - s t r u c t u r e d e t e r m i n a t i o n s ( E d w a r d s , 1 9 6 4 ) show t h a t n i o b i u m a n d t a n t a l u m pentafluorides exist as t e t r a - m e r s (Fig. 1 ) . As will b e seen, t h i s is a c o m m o n s t r u c t u r a l t y p e for pentafluorides; it contains t h e m e t a l a t o m s a t t h e corners of a s q u a r e a n d linked b y linearly b o n d e d fluorine a t o m s . T h e l a t t e r feature is observed in o t h e r fluorine-bridged s t r u c t u r e s , b u t n o theoretical t r e a t m e n t of t h e orbitals i n v o l v e d h a s y e t b e e n p r o d u c e d . A m o r e detailed discussion of t h e s t r u c t u r a l relationships a m o n g t h e p e n t a h a l i d e s will b e given later.
T A B L E I . P e n t a f l u o r i d e s o f v a n a d i u m , n i o b i u m , a n d t a n t a l u m
182 Α. D . BEVERIDGE AND H. C. CLARK
F i G . L Crystal structure of NbFg.
(ii) Pentachlorides of Niobium and Tantalum
More physical c o n s t a n t s h a v e b e e n d e t e r m i n e d a c c u r a t e l y for t h e s e t w o c o m p o u n d s t h a n for a n y o t h e r p e n t a h a l i d e s . T h e values are listed in T a b l e I I , a n d clearly t h e r e is still considerable u n c e r t a i n t y a b o u t some of t h e values. R e p o r t e d values for t h e melting p o i n t of NbClg r a n g e from 204-2° (Nisel'son et al., 1964b) t o 209-5° (Alexander a n d F a i r b r o t h e r , 1949b). A critical e x a m i n a t i o n t e n d s t o confirm t h e sugges
tion of Schafer a n d K a h l e n b e r g (1960) t h a t A l e x a n d e r a n d F a i r b r o t h e r ' s m e a s u r e m e n t s on b o t h NbClg a n d TaClg are systematically in error b y 5-6°, a n d T a b l e I I c o n t a i n s w h a t are considered t o be t h e m o s t reliable values. T h e liquid r a n g e for b o t h pentachlorides does n o t exceed 45°, a n d values of t h e T r o u t o n c o n s t a n t s strongly indicate some association in t h e liquid s t a t e . This is, however, less extensive t h a n in t h e corre
sponding m o l t e n pentafiuorides, since t h e l a t t e r h a v e viscosities 70-100 t i m e s greater t h a n t h o s e of t h e pentachlorides. Similarly, t h e degree of self-ionization of t h e pentachlorides m u s t be m u c h less t h a n for t h e pentafluorides, since t h e speciflc c o n d u c t a n c e of N b F g is a b o u t 70 times t h a t of NbClg, a n d t h e specific c o n d u c t a n c e of TaFg is a b o u t 50 t i m e s t h a t of TaClg.
Direct d e t e r m i n a t i o n s of t h e s t r u c t u r e s of t h e pentachlorides confirm t h a t t h e t e n d e n c y t o w a r d s association is less t h a n in t h e pentafluorides.
A n early electron diffraction s t u d y (Skinner a n d S u t t o n , 1940) of NbClg, NbBrg, TaClg, a n d TaBrg in t h e v a p o u r p h a s e gives results which are i n t e r p r e t e d in t e r m s of trigonal b i p y r a m i d a l molecular s t r u c t u r e s . H o w ever, in view of t h e refinements t h a t h a v e b e e n m a d e w i t h respect t o this t e c h n i q u e since t h a t t i m e , some r e - e x a m i n a t i o n would be desirable.
A m u c h l a t e r infrared a n d R a m a n spectroscopic s t u d y of NbClg ( G a u n t a n d Ainscough, 1957) also assumes t h a t t h e molecular species is m o n o meric a n d either trigonal b i p y r a m i d a l or s q u a r e p y r a m i d a l in s h a p e .
PENTAHALIDES OF THE TRANSITION METALS 183
T A B L E I I . P e n t a c h l o r i d e s o f n i o b i u m a n d t a n t a l u m
N b C L T a C l ,
M e l t i n g p o i n t ( ° C ) B o i l i n g p o i n t ( ° C ) T r i p l e p o i n t {°C) D e n s i t y ( g c m - ^ ) s o l i d ( X - r a y ) l i q u i d ( a t m . p . ) V i s c o s i t y ( c p o i s e ; a t m . p . ) C r i t i c a l t e m p e r a t u r e (°C) C r i t i c a l p r e s s u r e ( a t m ) C r i t i c a l d e n s i t y ( g c m - ^ ) S p e c i f i c c o n d u c t a n c e ( X 10» o h m- i c m- i ) C r y s t a l d a t a ( A )
AFL^°FORMATION ( k c a l m o l e - ^ ) ΔΗ°FORMATION (g) ( k c a l m o l e " ! ) ΔΊΊΝΑΡ ( k c a I m o l e- 1 )
A/SVAP (e.u.) ( T r o u t o n c o n s t a n t ) FUSION ( k c a l m o l e- i )
A^'FUSION (e.u.) A i T s u i D i ( k c a l m o l e - ^ ) AIÎHYDROIYSIS ( k c a l m o l e - ^ )
2 0 4 · 7 « 2 0 6 - 8 ± 0-3&
2 4 7 - 4 ± 0-1^
2 4 8 - 3 ± 0-25^
2 0 3 - 4 ± 0 - 2 4
2 - 8 4 / 2-0737^
0-921Λ 534*
0-46*
0·68«
0 - 2 2 ( a t 220-235°)^·
M o n o c l i n i c ^
a = 1 8 - 3 0 ; 6 = 1 7 - 9 6 ; c = 5 - 8 8 ; ή = 9 0 - 6 ° - 1 6 9 - 9 ^
1 2 - 6 ± 0-4^
1 3 - 0 ± 1-3^
25- O P 2 5 - 1 « 8-15'· 9-95«
2 0 - 0 ± 0-8^
- 6 5 - 5 9 ± 0 - 4 5 «
216-5«»'' 234-0 ± 0-25«
232-9 db 0-1^
215-9 ± 0-1^
3-76^
2-6840^^
1-003Λ 494^
0-43*
0-89Î 0-30 (at 230-240°)^
M o n o c l i a i c
( i n d i s t i n g u i s h a b l e i n s i z e f r o m NTbCls*)
-205-0," -205-5"*
-179-8,»*
- 1 8 2 - 7 0 13-1 ± 0-4^
25-9 ± 1-3^
8-4»· 9-15«
16 d= 2*
22-3,c 25-2" 22-7*
- 7 1 - 3 ± 0-2V
« S c h a f e r a n d P i e t r u c k ( 1 9 5 1 b ) .
^ M e y e r et al ( 1 9 6 1 ) .
c O p y k h t i n a a n d F l e i s h e r ( 1 9 3 7 ) . A i n s c o u g h et al. ( 1 9 5 7 ) .
« N i s e l ' s o n ( 1 9 5 8 ) .
/ D o u g l a s s a n d S t a r i t z k y ( 1 9 5 7 ) . R o l s t e n ( 1 9 5 8 ) .
Λ N i s e l ' s o n a n d P u s t i l ' n i k ( 1 9 6 3 ) .
* N i s e l ' s o n et al. ( 1 9 6 4 a ) . i B i l t z a n d V o i g t ( 1 9 2 1 ) .
^ Z a l k i n a n d S a n d s ( 1 9 5 8 ) .
I S c h a f e r a n d K a h l e n b e r g ( 1 9 6 0 b ) . G r o s s et al. ( 1 9 6 0 ) .
η S c h a f e r a n d K a h l e n b e r g ( 1 9 5 8 ) . ο S c h a f e r a n d K a h l e n b e r g ( 1 9 6 0 a ) . Ρ F a i r b r o t h e r a n d F r i t h ( 1 9 5 1 ) .
ί S c h a f e r et al. ( 1 9 5 2 ) . r V o i t o v i t c h , et al. ( 1 9 6 1 ) .
« N i s e l ' s o n a n d P e r e k h r e s t et al. ( 1 9 6 1 ) .
* S h c h u k a r e v a n d K u r b a n o v ( 1 9 6 2 ) . w S h c h u k a r e v et al. ( 1 9 6 0 a ) . V S h c h u k a r e v et al. ( 1 9 6 0 b ) .
Since t h e R a m a n s p e c t r u m w a s d e t e r m i n e d w i t h s o h d NbClg, a n d t h e infrared w i t h c a r b o n disulphide solutions, t h i s a s s u m p t i o n is of d o u b t ful v a l i d i t y , a n d i n fact is i n direct conflict w i t h a m o r e r e c e n t X - r a y d e t e r m i n a t i o n . Z a l k i n a n d S a n d s (1958) h a v e s h o w n t h a t crystalline n i o b i u m p e n t a c h l o r i d e consists of d i m e r s , NbgClio, w i t h t h e chlorine a t o m s forming t w o slightly d i s t o r t e d o c t a h e d r a w h i c h s h a r e a c o m m o n edge (Fig. 2). T h e n i o b i u m a t o m s o c c u p y t h e c e n t r e s of t h e o c t a h e d r a
184 Α. D. BEVERIDGE AND H. C. CLARK
F i G . 2. Crystal structure of t h e N b C l , d i m e r .
a n d are joined b y t w o bridging chlorine a t o m s . T h e Nb—CI bridge b o n d l e n g t h is 2-56 Â, while t h e N b — C I non-bridge b o n d l e n g t h s are 2-25 a n d 2-30 Â. I t is interesting t h a t NbFg a n d TaFg should exist in t h e solid s t a t e as t e t r a m e r s containing linear M—^F—M bridge b o n d s , while t h e pentachlorides occur only as dimers w i t h b e n t M — C l — b r i d g i n g bonds. This l a t t e r s t r u c t u r e contains t h r e e sets of n o n - e q u i v a l e n t chlorine a t o m s , b u t , p e r h a p s surprisingly, a nuclear q u a d r u p o l e resonance s t u d y of NbClg (Reddoch, 1961) showed only a single ^^Cl resonance, a n d t h u s did n o t differentiate b e t w e e n t h e t h r e e t y p e s of chlorine.
(iii) Pentabromides and Pentaiodides of Niobium and Tantalum These are coloured, crystalline solids, n i o b i u m p e n t a b r o m i d e being described as red, TaBrg as yellow, NbIg as b r o n z e w i t h a metallic lustre, a n d Talg as b r o w n t o black. T h e r e is still considerable u n c e r t a i n t y a b o u t their melting p o i n t s in p a r t i c u l a r . T h e t w o values for TaBrg (Alexander a n d F a i r b r o t h e r , 1949b; W i s e m a n a n d Gregory, 1949) a p p e a r e d simul
taneously in t h e l i t e r a t u r e a n d differ b y 15°, while a fairly r e c e n t r e p o r t (Nisel'son a n d P e t r u s e v i c h , 1960) s t a t e s t h a t n i o b i u m p e n t a i o d i d e does n o t m e l t b u t decomposes w i t h loss of iodine a t t e m p e r a t u r e s in excess of 280-300°. Confusion over t h e crystal d a t a for n i o b i u m p e n t a b r o m i d e has also only recently been r e m o v e d . Zalkin a n d S a n d s (1958) r e p o r t e d a monoclinic s t r u c t u r e isomorphous w i t h n i o b i u m p e n t a c h l o r i d e , while Rolsten (1958b) observed a n o r t h o r h o m b i c cell. A l a t e r critical e x a m i n a tion (Berdonosov et al., 1963) confirms t h e o r t h o r h o m b i c s t r u c t u r e giving t h e cell dimensions shown i n T a b l e I I I . Similarly, earlier r e p o r t s (Rolsten, 1959; D a h l a n d W a m p l e r , 1959) suggested t h a t n i o b i u m pentaiodide was also o r t h o r h o m b i c , b u t t h e m o r e detailed a n d m o r e recent s t u d y ( L i t t k e a n d B r a u e r , 1963) h a s led t o t h e monoclinic cell dimensions, cited in t h e t a b l e .
PENTAHALIDES OF THE TRANSITION METALS 185
TABLE I I I . P e n t a b r o m i d e s a n d p e n t a i o d i d e s o f n i o b i u m a n d t a n t a l u m
NbBrg TaBrg N b l g T a i s
Melting point (°C) 267·5« 265 dz 5& d > 280^ 496 ± 2^
280-0«
Boiling point (°C) 361-6 ± 0-l« 345&
348-8 ± 0-l« — 543 ± O-5'ί
Triple point (°C) 267-5 ± l-0« 267&
280-0 ± l-0« — —
D e n s i t y (g cm-^)
(X-ray) 5-24fl' 5-05/ 5-24Λ 5-809fl'
(pycn.) 4-36^./ = 4 - 9 8 9 ± 5 - 1 1 Λ 5-79fi^
0 - 0 0 3 /
Crystal d a t a (A) Ortho- Ortho- Monoclinic^ Ortho-
r h o m b i c / r h o m b i c / rhombic 17
a = 6-127 a = 6-155 a = 10-58 a = 6-65
b = 12-98 b = 13-29 b = 6-58 b = 13-95
c = 18-55 c = 18-66 c = 13-88 c = 20-1
β = 109°14' Δ ί ί ° formation - 1 3 5 - 2 i - 1 4 5 - 6 ^
(kcal m o l e - i ) -132-9*^ - 1 4 2 - 9 ' ^ — —
Affvap (kcal mole-^) 18-7« 14.9a,& — 18-1<*
AiSvap (Trouton const., 2 9 - 5 ^ 2 4 - P , 24-0»* — 2 2 - 2 ^
e.u.) 31-3^
ΔίΖ^fusion (kcal m o l e - i ) 9-2fc 10-9«'& — 1-6^
Δ/isubi (kcal m o l e - i ) — 25-8«.& — 19-7^*
Aiihydrolysls
(kcal mole~^) - 6 8 - 3 ± 0-9^ - 7 5 - 6 ± 0-3^ —
—
α Alexander and Fairbrother (1949b).
^ W i s e m a n and Gregory (1949).
c Nisel'son a n d Pustil'nik (1960), Alexander a n d Fairbrother (1949a).
« R o l s t e n (1958b).
/ B e r d o n o s o v et al. (1963) R o l s t e n (1958a).
Λ R o l s t e n (1959).
i L i t t k e a n d Brauer (1963).
^ Shchukarev et al. (1962).
* Gross et al. (1962).
ι Shchukarev et al. (1960b).
^ Fairbrother a n d Frith (1951).
T h e T r o u t o n c o n s t a n t values i n d i c a t e t h a t some association occurs in t h e liquid p e n t a b r o m i d e s , b u t n o v a l u e is available for n i o b i u m pentaiodide, a n d t h a t for t a n t a l u m p e n t a i o d i d e is i n t h e n o r m a l r a n g e for non-associated liquids. T h e m o d e of association in t h e solid com
p o u n d s m a y well b e t h e s a m e as in n i o b i u m p e n t a c h l o r i d e (i.e. dimeric units) b u t single crystal s t r u c t u r e d e t e r m i n a t i o n s are necessary for confirmation. (See also t h i s v o l u m e , p p . 123if.)
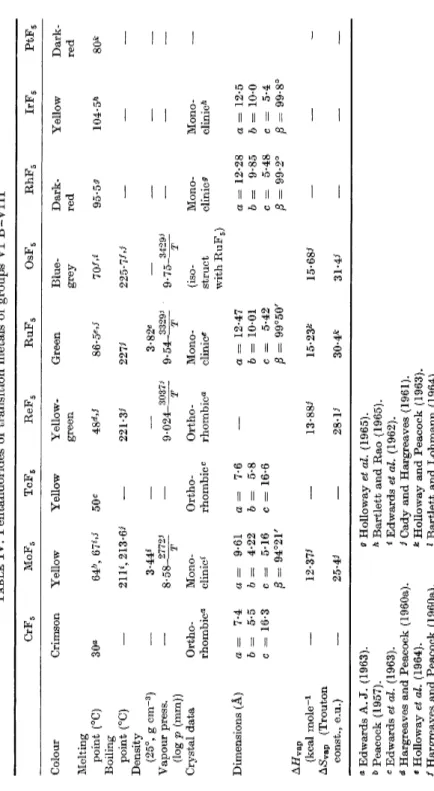
(iv) Pentafluorides of the Transition Metals of Groups VI Β-VIII This g r o u p of pentafluorides h a s e x p a n d e d r a p i d l y i n t h e last few years w i t h t h e a d d i t i o n of t h e pentafluorides of p l a t i n u m , iridium, m o l y b d e n u m , a n d r h o d i u m . M a n y physical c o n s t a n t s h a v e y e t t o b e d e t e r m i n e d , a n d t h e high reactivities of t h e c o m p o u n d s m a k e a c c u r a t e
TABLE IV. Pentafluorides of transition metals of groups VI Β -VIII 00 05 CrFg M0F5 TcFg ReFs RuFg OsFs PhFs IrF, PtFs Colour Crimson Yellow Yellow Yellow-Green Blue-Dark-Yellow Dark- green grey red red Melting point (°C) 64^ 67*·'^· 50c 48^.i 86-5^»^· 70/'*· 95-59^ 104-5^ 80fc Boiling point (°C)
—
21IÎ, 213-6^·—
221-3^· 227i 225-7/.:'·—
Density (25°, g cm~3)—
3-44Η —
3-82^— — —
Vapour press.—
8-58—
9.024-^037.' 9.54_3329. 9-75 ^429^'— — —
(log ρ (mm)) τ Τ τ Τ Crystal data OrthoMonoOrthoOrthoMono-(iso-MonoMono—
rhombic» clinic*" rhombic c rhombic'^ clinic« struct clinic^ clinic^ with RuFg) Dimensions (A) a = 7-4 a = 9-61 a = 7-6—
a = 12-47 a = 12-28 a = 12-5 6 = 5-5 6 = 4-22 b = 5-8 b = 10-01 b = 9-85 b = 10-0 c = 16-3 c = 5-16 c = 16-6 c = 5-42 c - 5-48 c = 5-4 ή = 94°2Ρ ή == 99°50' ή = 99-2° jS = 99-8° (kcal mole-^—
12-37Î — 13-88Î 15-23^ 15-68^— —
AiSvap (Trouton const., e.u.)—
25·4ί—
28· 30-4^^ 31-4^ —— —
ϋ % Α Q ϋ ρ «Edwards A.J. (1963). & Peacock (1957). c Edwards et al. (1963). ^ Hargreaves and Peacock (1960a). «HoUoway ei al. (1964). /Hargreaves and Peacock (1960a).
^HoUoway et al. (1965). Λ Bartlett and Rao (1965). «Edwards et al. (1962). ί Cady and Hargreaves (1961). * Hollo way and Peacock (1963). ' Bartlett and Lohmann (1964).
PENTAHALIDES OF THE TRANSITION METALS 187 physical m e a s u r e m e n t s v e r y difi&cult. As a class, t h e s e pentafluorides h a v e high volatility, w i t h melting p o i n t s below 100°, a n d boiling p o i n t s of 200-230°. T h e h e a t s of v a p o r i z a t i o n a n d t h e T r o u t o n c o n s t a n t values are all high, t h e l a t t e r p a r t i c u l a r l y so, indicating t h a t association occurs in t h e liquid s t a t e . T h e r e is n o direct evidence, however, t o confirm t h i s or t o p r o v i d e information on t h e degree or m o d e of association. T h e crystal t y p e a n d cell dimensions h a v e b e e n d e t e r m i n e d for t h e m a j o r i t y of t h e k n o w n pentafluorides of t h e s e m e t a l s , b u t only t w o crystal s t r u c t u r e s h a v e b e e n d e t e r m i n e d i n detail. M o l y b d e n u m pentafluoride was t h e first t r a n s i t i o n m e t a l pentafluoride for w h i c h t h e t e t r a m e r i c s t r u c t u r e of F i g . 1 w a s established ( E d w a r d s et al., 1962). R u t h e n i u m pentafluoride possesses a d i s t o r t e d version of t h e M0F5 s t r u c t u r e (HoUoway et al., 1964), in which t h e s t r u c t u r a l u n i t is still a t e t r a m e r . H o w e v e r , in c o n t r a s t t o t h a t of M0F5, t h e r u t h e n i u m a t o m s are a t t h e corners of a r h o m b u s w i t h non-linear R u — F — R u bridge b o n d s (Fig. 3).
F I G . 3. Crystal structure of RuFg.
O s m i u m a n d iridium pentafluorides are i s o m o r p h o u s w i t h r u t h e n i u m pentafluoride a n d p r e s u m a b l y c o n t a i n t h e s a m e t y p e of t e t r a m e r i c u n i t . O n t h e o t h e r h a n d , p r e l i m i n a r y X - r a y studies of t h e pentafluorides of c h r o m i u m , t e c h n e t i u m , a n d r h e n i u m h a v e s h o w n t h a t t h e y h a v e o r t h o r h o m b i c u n i t cells, a n d possibly h a v e s t r u c t u r e s different from either m o l y b d e n u m or r u t h e n i u m pentafluorides ( E d w a r d s , A. J . , 1963).
These s t r u c t u r a l relationships will b e discussed later, as will t h e m a g netic properties of t h e s e pentafluorides.
(v) Other Pentahalides of the Transition Metals of Groups VI B-VIII T h e t r a n s i t i o n m e t a l s of groups V I B - V I I I give few p e n t a h a l i d e s , other t h a n pentafluorides. T h e t h r e e well-characterized c o m p o u n d s are
188 Α. D . BEVERIDGE AND H. C. CLARK
t h e pentachlorides of m o l y b d e n u m a n d t u n g s t e n , a n d t u n g s t e n p e n t a bromide. R h e n i u m pentachloride is k n o w n a n d also i t s analogue, r h e n i u m p e n t a b r o m i d e , b u t few of their physical properties h a v e b e e n determined. Some of t h e k n o w n physical characteristics a r e p r e s e n t e d in T a b l e V.
T A B L E V . P h y s i c a l p r o p e r t i e s o f M0CI5, WCI5, WBr^, a n d R e C l g
M0CI5 WCI5 R e C l s
C o l o u r B l a c k D a r k g r e e n B l a c k w . g r e e n C i n n a
i r r i d e s c e n c e m o n
M e l t i n g p o i n t (°C) 194, ^ 1 9 0 « 2 3 0 ± 10« 2 8 6 d z 3^^ - 2 6 0 ^
B o i l i n g p o i n t (°C) 268^* 2 8 6 d z 2« 3 9 2 d z 2& - 3 3 0 c
D e n s i t y (g cm~^) 2 - 9 2 8 / —
— —
S p e c i f i c c o n d u c t a n c e . ( X 10-« o h m- i c m- i ) 7 - 5 ( a t 2 5 8 ° ) s ' l - 8 ( a t 2 1 6° ) 9 ' 0-67 ( a t 250°)^ 1-84 ( a t 300°)^
— —
——
C r y s t a l d a t a ( A ) M o n o c l i n i c /
— — •—
a = 1 7 - 3 1 b = 1 7 - 8 1 c = 6 - 0 7 9 β = 9 5 - 7 ° Δ ί ί ° formation
( k c a l m o l e- i ) - 1 2 6 - 6 (s)'^ - 1 3 7 (s)^ - 8 4 - 6 d z 1-2 (s)i —
Aif°formation
( k c a l mole~^) - 1 0 8 (g)^ - 1 1 9 (g)^ — —
AiS*"formation (e.u.) 77 (s)^ 71 (s)'^
—
—Aiifusion ( k c a l m o l e- 1 )
— —
5&—
AHyap ( k c a l mole~^) 13-9:' 13-6 ± 2h 14-5 d i 0-5&
—
A/S'vap ( T r o u t o n
c o n s t . , e.u.) 25-3^ 2 3 - 9 ± 1Λ 2 1 - 8 d : 0-5&
—
Aiisubi ( k c a l mole~^) 21-7^· 18-5 ± 0-5/^ 19-5 d : 1^
—
18-3 ± 0-5^
A^subi (e.u.) 43-5:^ 30-3« 3 6 - 6 d z 2&
—
3 5 - 6 ± 1Λ 33-1 ± O'lf^
a C o l t o n a n d T o m p k i n s (1965) f> S h c h u k a r e v et al. ( 1 9 5 9 b ) . ' ^ L e b e d e v ( 1 9 6 2 ) .
<i D e b r a y ( 1 8 6 8 ) .
« S h c h u k a r e v a n d N o v i k o v ( 1 9 5 6 ) .
/ S a n d s a n d Z a l k i n ( 1 9 5 9 ) . ff V o i g t a n d B i l t z ( 1 9 2 4 ) . Λ S h c h u k a r e v et al. ( 1 9 6 0 c ) .
i S h c h u k a r e v a n d K o k o v i n ( 1 9 6 4 ) . j S h c h u k a r e v a n d S u v o r o v ( 1 9 6 1 ) .
T h e c o m p o u n d s a r e intensely coloured solids, as would b e expected from t h e incomplete iî-electron shells of t h e m e t a l s concerned. Their melting a n d boiling points a r e quite high, a n d a r e n o t k n o w n accurately in certain cases. T h e T r o u t o n c o n s t a n t values give evidence of associa
tion i n t h e liquid s t a t e , a l t h o u g h t h e deviations from ' ' n o r m a l " values are n o t large except for m o l y b d e n u m pentachloride. T h e conductivities of m o l y b d e n u m a n d t u n g s t e n pentachlorides i n t h e m o l t e n s t a t e also
PENTAHALIDES OF THE TRANSITION METALS 189 indicate t h a t a small degree of auto-ionization occurs, a l t h o u g h t h i s c a n n o t b e extensive.
( M C l 5 ) „ ^
I
MCI4+ + ^ MCle-T h e t e n d e n c y for t h e m e t a l a t o m t o acquire a n e v e n co-ordination n u m b e r i n t h e solid s t a t e , is again s h o w n b y t h e crystal s t r u c t u r e of m o l y b d e n u m p e n t a c h l o r i d e (Sands a n d Zalkin, 1959). T h e monoclinic u n i t cell contains t w e l v e M0CI5 a r r a n g e d i n dimeric u n i t s , M 0 2 C I 1 0 . T w o chlorine b r i d g e - b o n d s u n i t e t h e t w o o c t a h e d r a l MoClg u n i t s . I t is i n teresting t h a t , a s for n i o b i u m p e n t a c h l o r i d e , electron diffraction d a t a for M0CI5 v a p o u r (Ewens a n d Lister, 1938) a r e b e s t i n t e r p r e t e d i n t e r m s of trigonal b i p y r a m i d a l m o n o m e r s . Again, however, some r e - e x a m i n a tion is desirable, p a r t i c u l a r l y since v a p o u r pressure m e a s u r e m e n t s of t u n g s t e n p e n t a c h l o r i d e ( S h c h u k a r e v et al,, 1959) indicate a dimeric species i n t h e v a p o u r .
(vi) Summary of Structures and Magnetic Properties
T h e s t r u c t u r e s so far d e t e r m i n e d for p e n t a h a l i d e s c a n b e usefully s u m m a r i z e d , b o t h i n t e r m s of s t r u c t u r a l t y p e s , a n d of t h e observed m e t a l - h a l o g e n distances, a s i n T a b l e V I . F i r s t l y , t h e fact t h a t aggrega
tions larger t h a n t h e dimers h a v e b e e n observed so far only for fluorides is w o r t h r e m a r k i n g . This m a y result from steric considerations, so t h a t a s t r u c t u r a l investigation of r h e n i u m p e n t a c h l o r i d e would h a v e g r e a t interest. Secondly, t h e m e t a l - h a l o g e n distances show several interesting features. I f t h e u s u a l values (Table of I n t e r a t o m i c Distances) for t h e covalent radii of fluorine a n d chlorine a r e applied t o t h e M — X (ter
minal) distances of n i o b i u m pentafluoride a n d p e n t a c h l o r i d e , values of
T A B L E V I . S t r u c t u r a l f e a t u r e s o f t h e p e n t a h a l i d e s C o m p o u n d
R e f . rM ( V )
T y p e o f s t r u c t u r a l u n i t
M e t h o d ( p h a s e )
M — X (A) ( b r i d g i n g )
M — X (A) ( t e r m i n a l )
N b F^ I
T a F g j a S q u a r e t e t r a m e r X - r a y ( s o l i d ) 2-06 ( l i n e a r b r i d g e ) 1-77 N b C l s b D i m e r X - r a y ( s o l i d ) 2-56 ( n o n - l i n e a r b r i d g e ) 2-27 M 0 F 5 c S q u a r e t e t r a m e r X - r a y ( s o l i d ) 2-06 ( l i n e a r b r i d g e ) 1-78
M0CI5 e D i m e r X - r a y ( s o l i d ) 2 - 5 3 2-24
M0CI5 d M o n o m e r E l e c t , d i f f .
( v a p o u r )
—
2-27R u F g f T e t r a m e r ( r h o m b u s ) X - r a y ( s o l i d ) 2*05 ( n o n - l i n e a r b r i d g e ) 1-90 α E d w a r d s (1964)
» Z a l k i n a n d S a n d s ( 1 9 5 8 ) . c E d w a r d s et al. ( 1 9 6 2 ) .
^ E w a n s a n d L i s t e r (1938)
« S a n d s a n d Z a l k i n ( 1 9 5 9 ) / H o U o w a y et al. ( 1 9 6 4 ) .
190 Α. D . BEVERIDGE AND H. C. CLARK
1 -28 Â a n d 1 -06 Â respectively are o b t a i n e d for t h e r a d i u s of t h e p e n t a - v a l e n t n i o b i u m a t o m , a n d a similar s e t of v a l u e s is o b t a i n e d for m o l y b d e n u m . T h e large difference p r e s u m a b l y indicates a b o n d order some
w h a t g r e a t e r t h a n 1 for t h e pentafluoride. T h e r e is also a n u n e x p e c t e d consistency, i n t h a t t h e difference b e t w e e n t h e M — X (bridging) a n d M — X (terminal) distances is regularly 0-30 Â , e x c e p t for r u t h e n i u m pentafluoride. T h a t t h i s difference should b e only 0-15 Â for t h e l a t t e r c o m p o u n d is n o t readily explained, since t h e s t r u c t u r a l t y p e s of R u F g a n d , s a y N b F g , a r e less different t h a n t h o s e of N b F g a n d NbClg. T h e difference of 0-3 Â for t e r m i n a l a n d bridging halogens is appreciable, b u t t h e relatively wide occurrence of t h e s e a n d o t h e r halogen-bridged s t r u c t u r e s , a n d t h e s y m m e t r i c a l position of t h e halogen i n b o t h linear a n d non-linear bridges clearly indicates t h e s t a b i l i t y of such s t r u c t u r e s . Finally t h e available d a t a for t h e m a g n e t i c properties of p a r a m a g n e t i c p e n t a h a l i d e s a r e given i n T a b l e V I I . I t is clear t h a t m a n y i m p o r t a n t m e a s u r e m e n t s still h a v e t o b e m a d e ; however, for cases w h e r e c o m p l e t e d a t a a r e available, t h e results a r e i n general a g r e e m e n t w i t h t h e t h e o retical predictions ( K o t a n i , 1949; E a r n s h a w et al., 1961), for t h e m a g netic b e h a v i o u r of t h e m e t a l a t o m s i n o c t a h e d r a l fields. F u r t h e r analysis m u s t a w a i t n e w m a g n e t i c a n d s t r u c t u r a l d a t a .
B. Preparation of the pentahalides
I n general t h e p e n t a h a l i d e s c a n b e o b t a i n e d b y direct c o m b i n a t i o n of t h e m e t a l a n d a p p r o p r i a t e halogen a l t h o u g h t h e conditions employed m a y v a r y widely. All t h e p e n t a h a l i d e s , however, a r e v e r y susceptible
T A B L E V I I . M a g n e t i c d a t a
C o m p o u n d M e t a l μ c a l e . (Β) / x e f f θ
c o n f i g . (Β a t 3 0 0 ° Κ ) ( W e i s s c o n s t a n t , ° K )
M o C l s »
ReCIg^^
ReFgC
RuFg^
O s F g i R h F / I r F /
d^
d^
d^
d^
1-73 2-83 2-83 3-88 3-88
2 - 8 3 ( f o r Î 2 a 0 2-83 ( f o r t^g*) 1-73 ( f o r Î 2, 6 )
1-67
2-21 a n d 2 - 5 7 1-41
3 · 3 - 3 · 6 2-31 2-95 1-32 p a r a m a g n e t i c
0
164 a n d 2 6 6 5 8 0
3 4
n o t e x t r a p . n o t e x t r a p . n o t e x t r a p .
« C o l t o n a n d T o m k i n s ( 1 9 6 5 ) .
^ K n o x a n d C o f f e y ( 1 9 5 9 ) . c H a r g r e a v e s a n d P e a c o c k ( 1 9 6 0 a ) .
^ H o l l o w a y a n d P e a c o c k ( 1 9 6 3 ) .
« H o l l o w a y et al. ( 1 9 6 5 ) ; B a r t l e t t ( p e r s o n a l c o m m u n i c a t i o n ) .
/ B a r t l e t t a n d R a o ( 1 9 6 5 ) ; B a r t l e t t ( p e r s o n a l c o m m u n i c a t i o n ) .
ff B r o w n a n d C o l t o n ( 1 9 6 4 ) . Λ B a r t l e t t a n d L o h m a n n ( 1 9 6 4 ) .
» H a r g r e a v e s a n d P e a c o c k ( 1 9 6 0 b ) .
PENTAHALIDES OF THE TRANSITION METALS 191
to hydrolysis, a n d in some cases also t o oxidation, so t h a t t h e rigorous exclusion of m o i s t u r e a n d o x y g e n is usually essential. T h e detailed p r o cedures so far r e p o r t e d a r e b e s t s u m m a r i z e d for each element.
(i) Vanadium Pentafluoride
A l t h o u g h t h i s c o m p o u n d w a s r e p o r t e d first b y Ruff a n d Lickfett (1911) t o b e p r o d u c e d b y t h e t h e r m a l d i s p r o p o r t i o n a t i o n of v a n a d i u m tetrafluoride, t h e first satisfactory p r e p a r a t i o n (Emeléus a n d G u t m a n n , 1949) r e q u i r e d t h e fluorination of v a n a d i u m m e t a l b y fluorine a t 300°.
This p r o c e d u r e h a s since b e e n described i n g r e a t e r detail (Clark a n d Emeléus, 1957). O t h e r m u c h less c o n v e n i e n t p r e p a r a t i v e m e t h o d s in
volve (a) t h e t h e r m a l d i s p r o p o r t i o n a t i o n of VF4 (Cavell a n d Clark, 1962), a n d t h e t h e r m a l decomposition a t 350° of p o t a s s i u m hexafluoro- v a n a d a t e ( V ) (Emeléus a n d G u t m a n n , 1949). A h e x a f l u o r o v a n a d a t e ( V ) , b u t n o t t h e pentafluoride itself, c a n b e o b t a i n e d b y t h e r e a c t i o n of v a n a d i u m ( V ) oxide w i t h n i t r y l fluoride (Aynsley et al., 1954).
(ii) Pentahalides of Niobium and Tantalum
P r e p a r a t i v e m e t h o d s for t h e s e p e n t a h a l i d e s h a v e b e e n discussed else
w h e r e in this v o l u m e b y F a i r b r o t h e r (p. 123fif) a n d need n o t b e r e p e a t e d here. T h e c o m p o u n d s are readily accessible a n d their p r e p a r a t i o n s b y direct c o m b i n a t i o n do n o t p r e s e n t a n y g r e a t difficulties. O t h e r syntheses involve t h e use of chlorine trifluoride (Nikolaev et al., 1958), h y d r o g e n fluoride (Nikolaev a n d Buslaev, 1959) or s u l p h u r tetrafluoride ( K e m m i t t a n d S h a r p , 1961) t o o b t a i n t h e pentafluorides, while t h e pentachlorides m a y a l t e r n a t i v e l y be p r e p a r e d from t h e reactions of n i o b i u m ( t a n t a l u m ) (V) oxide w i t h a l u m i n i u m trichloride (Chaigneau, 1956), w i t h t h i o n y l chloride ( F u n k a n d Weiss, 1958), or w i t h c a r b o n t e t r a c h l o r i d e (Ruff"
a n d T h o m a s , 1926) a l t h o u g h t h e last is suitable only for n i o b i u m p e n t a chloride. N i o b i u m t e t r a c h l o r i d e can also b e m a d e t o u n d e r g o dispro
p o r t i o n t o give t h e t r i - a n d pentachlorides (Schafer, 1955), a n d t h e s a m e is t r u e for t a n t a l u m t e t r a c h l o r i d e (Schafer a n d G r a u , 1954). T h e a c t i o n of h y d r o g e n chloride or h y d r o g e n b r o m i d e o n t a n t a l u m a t a p p r o x i m a t e l y 400° h a s also b e e n u s e d t o o b t a i n t h e p e n t a c h l o r i d e or p e n t a b r o m i d e ( Y o u n g a n d B r u b a k e r , 1952). T a n t a l u m p e n t a b r o m i d e h a s been o b t a i n e d from t h e p e n t o x i d e a n d c a r b o n t e t r a b r o m i d e , a l t h o u g h t h e related reaction w i t h n i o b i u m p e n t o x i d e gives niobium(V) o x y t r i bromide (Chaigneau, 1959). T h e p e n t a i o d i d e s of n i o b i u m a n d t a n t a l u m h a v e b e e n p r e p a r e d , o t h e r t h a n b y direct c o m b i n a t i o n , b y t h e a c t i o n of a l u m i n i u m ( I I I ) iodide on t h e p e n t o x i d e s (Chaigneau, 1957), or on t h e pentachlorides (Nisel'son a n d P e t r u s e v i c h , 1960).
192 Α. D. BEVERIDGE AND H. C. CLARK
(iii) Chromium Pentafluoride
V o n W a r t e n b e r g (1941) described t h e p r e p a r a t i o n of c h r o m i u m pentafluoride b y direct fluorination of t h e m e t a l . T h e reaction gives a v a r i e t y of fluorides, including t h e p e n t a - a n d tetrafluorides, a n d later work (Clark a n d S a d a n a , 1964) h a s confirmed t h e complexity a n d experi
m e n t a l difficulties of t h i s fluorination. T w o o t h e r r e c e n t b u t brief re
p o r t s describe (a) t h e difficulty of s e p a r a t i n g CrFg from CrOF4, b o t h of which are formed d u r i n g t h e fluorination ( E d w a r d s , A. J., 1963), a n d (b) t h e p r e p a r a t i o n of t h e pentafluoride t o g e t h e r w i t h c h r o m i u m h e x a fluoride in t h e high pressure (200 a t m ) , high t e m p e r a t u r e (400°) fluori
n a t i o n of c h r o m i u m (Glemser et aL, 1963). T h e only o t h e r r e p o r t e d r o u t e t o c h r o m i u m ( V ) fluoride leads t o its formation, along w i t h p o t a s sium hexafluorochromate(IV), in t h e direct fluorination of a m i x t u r e of p o t a s s i u m a n d c h r o m i u m ( I I I ) chlorides (Huss a n d K l e m m , 1950).
Certainly of t h e k n o w n p e n t a h a l i d e s of t h e first r o w t r a n s i t i o n m e t a l s , c h r o m i u m pentafluoride is t h e m o s t difficult t o p r e p a r e in a reasonable s t a t e of p u r i t y .
(iv) Molybdenum Pentafluoride
I n t h i s case, a l t h o u g h direct fluorination of m o l y b d e n u m p o w d e r a t 400° does yield t h e pentafluoride, t h e p r o d u c t is c o n t a m i n a t e d w i t h a large p r o p o r t i o n of t h e oxytetrafluoride from which complete separa
tion is v e r y difficult ( E d w a r d s et aL, 1962). More conveniently, t h e hexafluoride can b e r e d u c e d b y either m o l y b d e n u m or t u n g s t e n h e x a c a r b o n y l a t 25°, or b y m o l y b d e n u m p o w d e r a t 300-400°. T h e tetrafluoride is also formed in these reductions b u t h a s a m u c h lower volatility t h a n t h e pentafluoride which can therefore be readily purified.
An interesting a l t e r n a t i v e p r e p a r a t i o n of t h e pentafluoride requires t h e reaction of m o l y b d e n u m hexafluoride w i t h p h o s p h o r o u s trifluoride (O'Donnell a n d S t e w a r t , 1962): 2MoF6 + P F a - ^ P F s + M0F5.
(v) Technetium and Rhenium Pentafluorides
Again, direct fluorination leads t o these t w o pentafluorides. Fluorina
tion of t e c h n e t i u m m e t a l gives t h e hexafluoride, w i t h t h e pentafluoride a n d oxide tetrafluoride, TCOF4, formed as b y - p r o d u c t s which can b e reasonably s e p a r a t e d ( E d w a r d s et aL, 1963). T h e p r e p a r a t i o n of r h e n i u m pentafluoride requires m o r e precisely controlled conditions, since t h e p r o d u c t s of t h e reaction of r h e n i u m hexafluoride w i t h t u n g s t e n h e x a c a r b o n y l are d e t e r m i n e d b y t h e r a t i o of t h e r e a c t a n t s . W i t h a large excess of ReFg, t h e p r o d u c t s are r h e n i u m oxytetrafluoride (ReOF4), t u n g s t e n hexafluoride, a n d a residue whose composition a p p r o a c h e s t h a t of r h e n i u m tetrafluoride. W i t h a small excess of r h e n i u m h e x a fluoride, t h e pentafluoride a n d i m p u r e tetrafluoride were obtained, a n d
PENTAHALIDES OF THE TRANSITION METALS 1 9 3
with the same ratio of reactants dissolved in a fivefold excess of tungsten hexafluoride, the rhenium pentafluoride was the only major product (Hargreaves and Peacock, 1960a).
(vi) Ruthenium Pentafluoride
Two methods of preparation have been reported, both starting from the metal. The flrst involves direct fluorination of the metal (Ruff and Vidic, 1925), and this is best carried out at approximately 300° in a nickel tube, with the fluorine carried on a slow stream of nitrogen (HoUoway and Peacock, 1963). The pentafluoride is the only product and is readily separated b y distillation from unreacted metal and any ruthenium dioxide. The alternative preparation utilizes bromine tri
fluoride to fluorinate the metal, but this is a less satisfactory process (Hepworth et aL, 1954a).
(vii) Osmium Pentafluoride
As for other cases where the metal forms a stable hexafluoride, the pentafluoride can conveniently be reached b y reduction of the hexa
fluoride. This can be brought about using tungsten carbonyl with excess of the hexafluoride, or with iodine in iodine pentafluoride solution, pro
vided osmium hexafluoride is present in excess over the iodine. Finally, irradiation of the hexafluoride with ultraviolet light at 25° gives the solid pentafluoride and free fluorine (Hargreaves and Peacock, 1960b).
Of these methods probably the latter is most convenient, although con
tinuous removal of the fluorine is necessary, since in a closed vessel the system appears to reach a stationary state.
(viii) Rhodium and Iridium Pentafluorides
The preparation of these two compounds has been described only briefly. Early reports (Ruff and Ascher, 1929) had suggested the pos
sible existence of RhFs, and this was also indicated by the observation (Chernick et aL, 1961) that rhodium hexafluoride, in its decomposition at room temperature, liberates half a mole of fluorine for each mole of hexafluoride decomposed. The preparation of the pentafluoride has now been reported (HoUoway et al,, 1965) and involves the action of gaseous fluorine at 90 lb in-^ on the trifluoride at 400°. The same authors have also identified the pentafiuoride, along with the hexa- and tetrafluorides, as a product of the combustion of rhodium wire in fluorine.
Iridium pentafluoride has been prepared b y the reaction of iridium metal with fluorine in the stoichiometric ratio at 350-380°. The penta
fluoride is best condensed as it is formed on the cooled portion of the metal reaction vessel (Bartlett and Rao, 1965). Re-examination b y these authors has also shown that the previously reported tetrafluoride
194 Α. D . BEVERIDGE AND H. C. CLARK
(Robinson a n d W e s t l a n d , 1956) is in fact t h e pentafluoride, a n d t h a t t h e r e d u c t i o n of iridium hexafluoride w i t h sihca a p p a r e n t l y proceeds according t o t h e e q u a t i o n
4 IrFg + Si 4 IvF, + SiF^
(ix) Platinum Pentafluoride
This can be p r e p a r e d ( B a r t l e t t a n d L o h m a n n , 1964) b y direct fluori
n a t i o n of p l a t i n u m ( I I ) chloride in a p l a t i n u m b o a t w i t h a 3:1 m i x t u r e of fluorine a n d nitrogen. I t can be fairly readily s e p a r a t e d from o t h e r p r o d u c t s which include dioxygenyl hexafluoroplatinate(V), a l t h o u g h m o r e vigorous conditions could lead t o t h e c o n s u m p t i o n of p l a t i n u m m e t a l a n d t h e co-formation of p l a t i n u m hexafluoride.
(x) Molybdenum Pentachloride
M a n y p r e p a r a t i o n s (e.g. K n o x a n d Coffey, 1959) of m o l y b d e n u m p e n t a c h l o r i d e h a v e b e e n described, all employing direct chlorination of t h e m e t a l a t m o d e r a t e l y high t e m p e r a t u r e s (e.g. 300°). T h e m o s t r e c e n t work (Colton a n d T o m k i n s , 1965), however, h a s s h o w n t h a t , a l t h o u g h direct chlorination does give excellent yields of t h e pentachloride, t h e p r o d u c t m a y c o n t a i n u p t o 5 % of m o l y b d e n u m oxide t e t r a c h l o r i d e as i m p u r i t y . This p r e s u m a b l y arises because of t h e oxide coating which c a n n o t be r e m o v e d completely e v e n b y d r y i n g t h e m e t a l in a flow of n i t r o g e n a t 200°. H o w e v e r , t h e oxide t e t r a c h l o r i d e can be sublimed o u t a t 80-90°, leaving p u r e black crystals of t h e pentachloride.
A n a l t e r n a t i v e synthesis of m o l y b d e n u m pentachloride uses t h e m e t a l oxide, t o g e t h e r w i t h c a r b o n t e t r a c h l o r i d e as t h e chlorinating a g e n t ( K n o x et al., 1957). T h e reaction is carried o u t in a m e t a l b o m b a t 400°. More recently, t h e s a m e t e c h n i q u e h a s been applied t o allow t h e use of m o l y b d e n u m a n d o t h e r m e t a l sulphides as t h e s t a r t i n g m a t e r i a l s for m o l y b d e n u m p e n t a c h l o r i d e a n d o t h e r chlorides (Bardawil et al,, 1964). T h i o n y l chloride h a s also been used as a chlorinating a g e n t t o give yields of M0CI5 as high as 7 0 % from t h e trioxide (Seifert a n d Q u a k , 1961).
(xi) Tungsten Pentachloride and Pentabromide
M e t h o d s used t o p r e p a r e these t w o t u n g s t e n p e n t a h a l i d e s generally m a k e use of either t h e instability of or direct r e d u c t i o n of t h e corre
sponding hexahalides. A tyjoical p r e p a r a t i o n of t h e pentachloride (Allen et al., 1964) involves r e d u c t i o n of t h e hexachloride w i t h h y d r o g e n a t 380-400°, a n d purification b y r e p e a t e d sublimation in nitrogen. A n o t h e r procedure (Novikov et al., 1961) employs r e d p h o s p h o r u s t o b r i n g a b o u t reduction of t h e hexachloride a t 250-280°.
3 WCle + P r e d -> 3 WCI5 + PCI3
PENTAHALIDES OF THE TRANSITION METALS 195
T h e p e n t a b r o m i d e h a s b e e n p r e p a r e d b y direct b r o m i n a t i o n of t u n g s t e n p o w d e r . T w o procedures h a v e b e e n described, differing in w h e t h e r or n o t t h e m i x t u r e of WBrg a n d WBr^ is a c t u a l l y s e p a r a t e d . B r o m i n a t i o n of t h e p o w d e r e d m e t a l using b r o m i n e v a p o u r d i l u t e d w i t h n i t r o g e n a t 1000° provides a one-step synthesis (Allen et al., 1964).
A l t e r n a t i v e l y , b r o m i n a t i o n a t lower t e m p e r a t u r e s gives a m i x t u r e of WBrg a n d WBrg which, w h e n sublimed a t 250-300° u n d e r a w o r k i n g v a c u u m , gives p u r e WBrg a n d b r o m i n e (McCarley a n d B r o w n , 1964).
A n earlier m e t h o d involves b r o m i n a t i o n of t u n g s t e n h e x a c a r b o n y l ( S h c h u k a r e v et al., 1959b) w i t h e l e m e n t a r y b r o m i n e . A vigorous re
action occurs even below 0° t o give t h e h e x a b r o m i d e , which on h e a t i n g in v a c u o a t 250° gives b r o m i n e , a n d t h e p e n t a b r o m i d e sublimes t o give black crystals w i t h a greenish iridescence.
(xii) Rhenium Pentachloride
A detailed p r o c e d u r e for t h e p r e p a r a t i o n of ReClg b y direct chlorina
t i o n of t h e m e t a l w a s described some t i m e ago ( H u r d a n d B r i m , 1939).
Commercial r h e n i u m m e t a l p o w d e r is used a t 500°, w i t h v e r y p u r e r h e n i u m a n d oxygen-free chlorine, or if higher t e m p e r a t u r e s a r e used, a m i x t u r e of ReClg a n d ReClg, or j u s t ReClg is o b t a i n e d . Chlorination of t h e sulphides, RcgSy (Glukhov et al., 1961), or ReSg (Tronov et al.,
1958) h a v e also b e e n used t o give t h e p e n t a c h l o r i d e . A l t e r n a t i v e l y , t h e general p r o c e d u r e described b y K n o x et al. (1957), b a s e d on high t e m p e r a t u r e chlorination using c a r b o n t e t r a c h l o r i d e , m a y be employed.
(xiii) Rhenium Pentabromide
R h e n i u m p e n t a b r o m i d e is p r e p a r e d as a bluish-green c o m p o u n d , which gives a d e e p blue v a p o u r , b y t h e direct b r o m i n a t i o n of metallic r h e n i u m a t 650° (Colton, 1962).
C. Chemical Properties
(i) Stability and Decomposition Products
T h e p e n t a h a l i d e s , as a class, are stable in t h e sense t h a t u n d e r ' ' n o r m a l " conditions, s p o n t a n e o u s decompositions do n o t generally occur. Stability, however, is a notoriously difficult t e r m t o employ, a n d for p r e s e n t purposes, since precise d a t a are lacking in m a n y cases, we will confine ourselves t o t h e r m a l stability.
T h e p e n t a h a l i d e s of v a n a d i u m , n i o b i u m a n d t a n t a l u m h a v e con
siderable stability, as d e m o n s t r a t e d b y t h e i r r e a d y s u b l i m a t i o n w i t h o u t decomposition, a l t h o u g h for all e x c e p t t h e pentafiuorides t h i s m u s t b e d o n e in a n a t m o s p h e r e of t h e a p p r o p r i a t e halogen.
196 Α. D. BEVERIDGE AND H. C. CLARK
F o r t h e pentafluorides of v a n a d i u m , n i o b i u m a n d t a n t a l u m , m e a g r e q u a n t i t a t i v e information concerning stability is available. T h e h e a t of formation of v a n a d i u m pentafluoride (Table I, p . 181) is quite high (Cavell a n d Clark, 1963b) a n d calculations show t h a t t h e decomposition
V F 5- > V F , + I F ^
is e n d o t h e r m i c t o t h e e x t e n t of 31 kcal mole"^. Similar values a r e n o t available for N b F g or TaFg, b u t in general these t h r e e pentafluorides a r e t h e r m a l l y stable t o q u i t e high t e m p e r a t u r e s , a n d p r o b a b l y are e v e n t u a l l y decomposed as a consequence of their fluorinating action o n t h e container materials, r a t h e r t h a n as a result of i n h e r e n t t h e r m a l instability.
N o t u n e x p e c t e d l y , since t h e m e t a l s are i n t h e i r m o s t c o m m o n oxida
t i o n s t a t e , t h e pentachlorides of n i o b i u m a n d t a n t a l u m also possess high t h e r m a l stability. B r e a k d o w n t o lower chlorides c a n only b e achieved, w i t h a n y readiness, in t h e presence of reducing a g e n t s . H o w ever, a value of —33-6 kcal mole"^ h a s b e e n given ( S h c h u k a r e v a n d K u r b a n o v , 1962) for for t h e decomposition of t a n t a l u m p e n t a chloride
TaCl5(g)-^TaCl4(g) + i C l ,
w i t h a value of 31-3 e.u. for Δ/S. T h e p e n t a b r o m i d e s of n i o b i u m a n d t a n t a l u m , for which t h e h e a t s of formation are of t h e order of 130-140 kcal mole~^, also h a v e reasonable t h e r m a l stability, b u t can be r e d u c e d b y a v a r i e t y of reducing a g e n t s . T h e pentaiodides h a v e lower stabilities.
I t is k n o w n t h a t t h e p r o d u c t s of t h e direct iodination of n i o b i u m m e t a l b y iodine v a p o u r m a y be NbIg, Nbl4, or Nblg.g, d e p e n d i n g on t h e t e m p e r a t u r e of t h e n i o b i u m (Chizhikov a n d Grinko, 1959). K o r o s y (1939) n o t e d t h a t N b l g decomposed a t 300-400° u n d e r v a c u u m t o give com
positions i n t h e r a n g e of Nblg-aa t o Nbl2.5, a n d this t h e r m a l decom
position h a s been used b y l a t e r workers (Corbett a n d S e a b a u g h , 1958) t o p r e p a r e Nbl4, NbIg, a n d m o r e recently Nbgig (Seabaugh a n d Corbett, 1965). T h e ease of t h e r m a l b r e a k d o w n of t a n t a l u m p e n t a i o d i d e is v e r y similar, a l t h o u g h t h e solid phases p r o d u c e d on decomposition are n o t entirely analogous t o those o b t a i n e d from Nblg.
T h e r e is v e r y little information available concerning t h e t h e r m a l stabilities of t h e pentafluorides of c h r o m i u m , m o l y b d e n u m , t e c h n e t i u m , r h e n i u m , r u t h e n i u m , osmium, r h o d i u m , iridium, a n d p l a t i n u m , a l t h o u g h all t h e melting p o i n t s a n d t h e boiling points of several are k n o w n (Table I V , p . 186). I n m a n y cases, t h e p r o b l e m of t h e r e a c t i v i t y of t h e pentafluoride w i t h t h e glass container obscures d i s p r o p o r t i o n a t i o n or decomposition reactions. I n o t h e r cases, t h e instability of t h e