Myocardial Postconditioning Is Lost in Vascular Nitrate Tolerance
Veronika Fekete, MD,* Zsolt Murlasits, PhD, † Eda Aypar, BPharm, MSc, PhD,*
Péter Bencsik, MD, PhD, † Márta Sárközy, MD,* Gábor Szénási, MD, PhD, ‡ Péter Ferdinandy, MD, PhD, MBA, † § and Tamás Csont, MD, PhD*
Abstract: Organic nitrates play an important role in the therapy of ischemic heart disease; however, their clinical application is limited by the development of vascular nitrate tolerance. We have previously shown attenuation of the cardioprotective effect of preconditioning in vascular nitrate tolerance. Here, we studied whether the development of vascular nitrate tolerance affects the infarct size, limiting effect of ischemic postconditioning (IPost) in the myocardium, and whether the activation of survival kinases plays a role in the molecular mechanism of postconditioning in the presence or absence of vascular nitrate tolerance.
Male Wistar rats were treated with nitroglycerin/vehicle for 3 days to induce vascular nitrate tolerance. On the fourth day, isolated hearts were subjected to 30-minute coronary occlusion followed by 120-minute reperfusion with or without IPost. In nontolerant hearts, postcondition- ing significantly decreased infarct size as compared with ischemia/
reperfusion; however, postconditioning failed to decrease infarct size in hearts of nitrate tolerant rats. Phosphorylation of ERK 1/2, Akt, or endothelial nitric oxide synthetase showed no significant differences between the groups at the 10th minute of reperfusion. Vascular nitrate tolerance interferes with the infarct size limiting effect of IPost.
Activation of survival kinases is not crucial in the molecular mechanism of postconditioning, which remains unaffected in nitrate tolerance.
Key Words:nitrate tolerance, nitroglycerin, infarct size, postcondi- tioning, survival kinase, RISK pathway
(J Cardiovasc PharmacolÔ2013;62:298–303)
INTRODUCTION
Ischemic heart disease is the leading cause of death in industrialized countries. The mortality of acute myocardial
infarction can be decreased by procedures that allow the rapid return of blood flow to the ischemic myocardium, that is, reperfusion therapy. After the induction of endogenous pro- tective mechanisms, the myocardium is able to adapt to ischemia/reperfusion (I/R) stress. Ischemic preconditioning is a well-described adaptive response in which brief exposure to I/R markedly enhances the ability of the myocardium to withstand a subsequent injury.1,2However, the clinical applica- bility of ischemic preconditioning is limited. Conversely, ische- mic postconditioning (IPost), that is, brief cycles of I/R applied after a longer period of ischemia, have been shown to reduce myocardial damage in both animal studies and patients.3–5The precise mechanism of myocardial postconditioning is not entirely clear. However, activation of Reperfusion Injury Sal- vage Kinase (RISK) pathway has been proposed to be a possible mechanism for cardioprotection elicited by ischemic precondi- tioning and IPost.6–8 Nevertheless, accumulating evidence shows that several risk factors and pathological conditions, including hyperlipidemia,9 diabetes,10 and statin exposure,11 may interfere with these cardioprotective mechanisms.1
Organic nitrates have been used for the prevention and treatment of ischemic heart diseases for more than 100 years.12,13The main limitation of long-term nitrate therapy is the development of vascular nitrate tolerance, which leads to the abolishment of clinical or hemodynamic response (ie, vasodilatation with a subsequent decrease in blood pressure or relief in anginal pain) to organic nitrates after prolonged or high-dose nitrate treatment.12 We have previously reported that the cardioprotective effect of pacing-induced precondi- tioning is lost in the presence of vascular nitrate tolerance.14 Other groups have shown that the presence of vascular nitrate tolerance aggravated postischemic myocardial apoptosis and also impaired cardiac functional recovery after ischemia.15In a recent human study, Gori et al16 have reported that the endothelial preconditioning effect of a single dose of nitro- glycerin is lost upon a prolonged exposure to nitroglycerin.
However, whether postconditioning is affected by nitrate pre- treatment leading to the development of vascular nitrate tol- erance remains to be elucidated.17
Therefore, the aim of our present study was to examine whether the development of vascular nitrate tolerance affects the infarct size limiting effect of IPost in the myocardium, and whether the activation of RISK pathway plays a substantial role in the molecular mechanism of the cardioprotective effect of IPost in the presence or absence of vascular nitrate tolerance.
Received for publication March 14, 2013; accepted April 28, 2013.
From the *Cardiovascular Research Group, Department of Biochemistry, Uni- versity of Szeged, Hungary; and†Pharmahungary Group, Szeged, Hun- gary; and Departments of ‡Pathophysiology and §Pharmacology and Pharmacotherapy, Semmelweis University, Budapest, Hungary.
Supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by grants from the Hungarian Scientific Research Fund (K79167) and National Development Agency (MED_FOOD, TÁMOP-4.2.1/B-09/1/
KONV-2010-0005, TÁMOP-4.2.2/B-10/1-2010-0012, TÁMOP-4.2.2.A-11/
1/KONV-2012-0035).
E. Aypar was a visiting fellow from Gazi University, Ankara, Turkey, and her visit was supported by the Hungarian Scholarship Board.
The authors report no conflicts of interest.
Reprints: Tamás Csont, MD, PhD, Cardiovascular Research Group, Depart- ment of Biochemistry, University of Szeged, Dóm tér 9, Szeged H-6720, Hungary (e-mail: csont.tamas@med.u-szeged.hu).
Copyright © 2013 by Lippincott Williams & Wilkins
METHODS Ethics Statement
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (publication No. 85-23, revised 1996) and was approved by the Committee on Animal Experiments of the University of Szeged and the Csongrád County Animal Health and Food Control Station.
Induction and Verification of Vascular Nitrate Tolerance
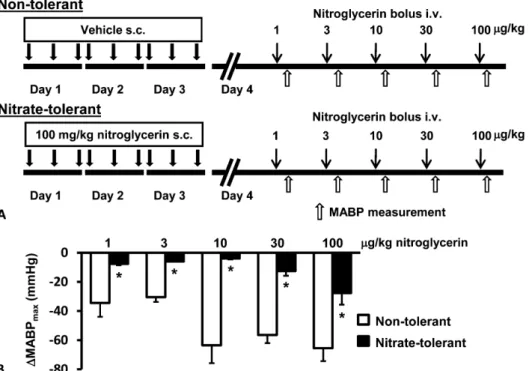
Male Wistar rats (300–350 g) purchased from Charles- River Laboratories Hungary, Ltd (Isaszeg, Hungary) were housed in a room maintained at 12-hour light–dark cycles and at a constant temperature of 226 28C. The animals had free access to standard laboratory chow and drinking water. Rats were treated with nitroglycerin (100 mg/kg subcutaneously;
EGIS Pharmaceuticals, Budapest, Hungary) or its vehicle lac- tose (900 mg/kg subcutaneously; Spektrum 3D, Debrecen, Hun- gary) 3 times a day for 3 days (9 repetitive injections) to induce vascular tolerance to nitroglycerin (n = 3 in both groups), as described in previous studies18–20(Fig. 1A). On the fourth day, approximately 12 hours after the last injection, rats were anes- thetized by intraperitoneal injection of 60 mg/kg pentobarbital (Euthanyl; Bimeda-MTC Animal Health, Inc, Cambridge, Can- ada). The adequacy of anesthesia was confirmed by monitoring heart rate, arterial blood pressure, and the lack of withdrawal response to a toe or tail pinch, which are the commonly used methods of monitoring the depth of anesthesia in rats. When required additional doses of 10 mg/kg sodium pentobarbital was given to the animals. Surface ECG was monitored throughout the experiment (Experimetria Ltd, Budapest, Hungary), and body temperature was maintained at 378C using a heating
pad. Trachea was intubated with a cannula connected to a rodent ventilator (Harvard Apparatus, Holliston, MA). The animals were ventilated with room air at a frequency of 50–55 per mi- nute with 2.5-mL volume. Carotid artery was cannulated, and a small polyethylene catheter was inserted into the artery to measure arterial blood pressure (Haemosys; Experimetria, Ltd, Budapest, Hungary). Jugular vein was cannulated for intrave- nous drug administration.21After stabilization of blood pressure, increasing doses of nitroglycerin (Nitro-POHL; G. Pohl- Boskamp GmbH & Co, KG, Hohenlockstedt, Germany) were given intravenously (1, 3, 10, 30, and 100mg/kg) to verify the development of vascular nitrate tolerance. Each dose was given after normalization of blood pressure after the previous nitro- glycerin dose (;10 minutes between each dose). Maximal decrease in blood pressure was evaluated as maximal response to nitroglycerin.
Experimental Design and Ex Vivo Infarct Size Measurements
Vascular nitrate tolerance was induced in male Wistar rats (300–350 g, n = 36) as described above (Fig. 2A). On the fourth day, approximately 12 hours after the last injection of nitroglycerin or lactose, anesthetized rats were heparinized (500 U/kg heparin intravenously, Heparibene-Na; Merckle GmbH, Ulm, Germany). Hearts were then excised and subjected to ex vivo perfusion with Krebs–Henseleit solution according to Langendorff at a constant pressure of 100 cm H2O and a con- stant temperature of 378C, as described previously.22 During the 15-minute equilibration period, a silk suture was placed around the left anterior descendent coronary artery. Hearts were then subjected to 30-minute regional ischemia by the occlusion of the left anterior descendent coronary artery and then reper- fused for 120 minutes. In the postconditioning groups, post- conditioning was induced by 6 cycles of 10-second reperfusion
FIGURE 1. Validation of the devel- opment of vascular nitrate tolerance.
A, Experimental protocol. Various intravenous doses of nitroglycerin were separated by approximately 10-minute intervals. B, Maximal decrease of mean arterial blood pressure (MABP) after nitroglycerin injection. Results are expressed as mean 6 standard error of mean.
Repeated measures 2-way analysis of variance. *P , 0.05 versus non- tolerant rats (n = 3 in each group).
followed by 10-second ischemia applied at the onset of reper- fusion.11Coronary flow was measured before ischemia. Elec- trocardiogram was monitored throughout the experiment (Isosys; Experimetria Ltd). At the end of reperfusion, the cor- onary artery was reoccluded, and the heart was stained with 0.1% Evans blue for the determination of risk area. Hearts were then frozen, cut into slices, and stained with 1% triphenyltetra- zolium chloride dissolved in phosphate buffer (pH 7.4) for infarct size determination.9,23 Hearts were then fixed in 10%
formaldehyde and scanned. Infarct size was evaluated using a planimetry software (Infarct Size 2.4.b; Pharmahungary Group, Szeged, Hungary). Infarct size was expressed as the percentage of risk area.
In separate groups of animals (n = 32), nitrate tolerance was induced, and hearts were perfused using the same treatment and perfusion protocols, as described above. In the 10th minute of reperfusion, the anterior wall of the left ventricle was frozen for Western blot analysis, as described in previous studies.24
Western Blot Analysis
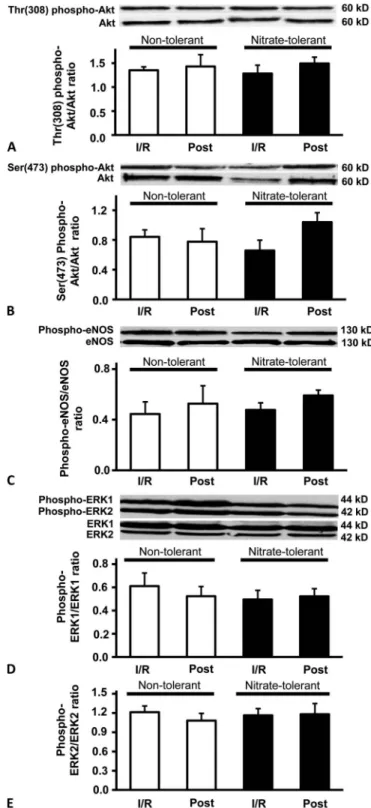
To examine the role of the activation of RISK pathway in IPost, we examined the activation of extracellular signal- related kinase (ERK) pathway investigating the phosphoryla- tion of 42/44 kDa ERK (ERK 1/2) and the activation of phosphoinositide-3 kinase/protein kinase B (PI3K/PKB/Akt) pathway measuring the phosphorylation of Akt and its downstream target endothelial nitric oxide synthetase (eNOS) in the ischemic zone of the left ventricle. Standard Western
blot analysis was performed, as described previously.25Tissue samples were homogenized in homogenization buffer [HEPES (10 mM), sucrose (0.32 mM), Na2EDTA (0.1 mM), dithiothreitol (1.0 mM), trypsin inhibitor (10mg/mL), leupeptin (10mg/mL), aprotinin (2.1mg/mL), Na vanadate (2.5 mM), NaF (2.5 mM), pH 7.4] and a serine protease inhibitor phenylmethanesul- fonylfluoride, and homogenates were centrifuged at 9000g (10 minutes, 48C). After determination of protein concentration of the supernatant by bicinchoninic acid assay (Pierce, Rock- ford, IL), sodium dodecyl sulphate polyacrylamide gel electro- phoresis (10% polyacrylamide gel, 90 V, 1.5 hours) was performed followed by transfer of proteins onto a nitrocellulose membrane (35 W, 2.5 hours; Amersham Biosciences, Piscat- away, NJ). Membranes were then blocked overnight at 48C in 5% milk dissolved in Tris-buffered saline containing 0.1%
Tween-20 and phosphatase inhibitor Na vanadate (1.0 mM).
Membranes were then incubated with anti–phospho (pSer473)- Akt (1:1000, Cell Signaling, Danvers, MA), anti–phospho (pThr308)-Akt (1:1000, Cell Signaling), anti-Akt (1:1000, Cell Signaling), anti–phospho-ERK 1/2 (1:1000, Cell Signal- ing), or anti–ERK 1/2 (1:1000, Cell Signaling) for 20 hours at 48C; or incubated with anti–phospho-eNOS (1:1000; BD Transduction Laboratories, Lexington, KY) or anti-eNOS (1:1000; BD Transduction Laboratories) primary antibodies at room temperature for 3 hours followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit (1:5000;
DakoCytomation, Glostrup, Denmark) or rabbit anti-mouse (1:5000; DakoCytomation) secondary antibodies for 40 minutes, respectively. Membranes were then developed with an enhanced chemiluminescence kit (Pierce), exposed to x-rayfilm and scanned. Band density was calculated by integrating the area (in pixel intensity, expressed in arbitrary units) using Quantity One evaluation software (Bio-Rad, Hercules, CA) and represented as a ratio of the phosphorylated and nonphos- phorylated proteins.
Statistical Analysis
Results are expressed as mean6standard error of mean.
Repeated measures 2-way analysis of variance followed by Bon- ferroni post hoc test and 2-way analysis of variance followed by Tukey post hoc test was used to evaluate differences in mean values between groups as appropriate. Differences were consid- ered significant atP, 0.05.
RESULTS
Induction and Verification of Vascular Nitrate Tolerance
To verify the development of vascular nitrate tolerance, we examined the vascular response to bolus injections of increasing doses of nitroglycerin by continuous monitoring of blood pressure. There was no difference between nontolerant and nitrate tolerant rats in basal hemodynamic parameters such as systolic, diastolic, and mean arterial blood pressure and heart rate (data not shown). The maximal decrease in mean arterial blood pressure was significantly lower in the nitrate tolerant animals after bolus injections of 3, 10, 30, 100, and 300mg/kg FIGURE 2. A, The experimental protocol for isolated heart
perfusion. B, The size of infarction after 30 minutes of regional ischemia and 120 minutes of reperfusion. Infarct size was normalized to the area at risk (AAR). Results are expressed as mean6standard error of mean. Two-way analysis of variance.
*P ,0.05 versus nontolerant I/R (n = 8–10 animals in each group). Post, postconditioning.
nitroglycerin as compared with nontolerant rats (Fig. 1B), indi- cating the development of vascular nitrate tolerance.
Effect of Vascular Nitrate Tolerance on IPost
To examine whether chronic nitrate treatment interferes with myocardial stress adaptation, we examined the infarct size limiting effect of IPost in the presence or absence of vascular nitrate tolerance. There was no significant difference in basal coronaryflow between the groups (data not shown).The different treatments had no significant effect on the area at risk. Infarct size was decreased by IPost in hearts of nontolerant rats compared with I/R group (Fig. 2B). Con- versely, IPost did not decrease infarct size significantly in the hearts of nitrate tolerant rats, and infarct size in nitrate tolerant rat hearts did not show significant difference com- pared with the nontolerant control group.
Effect of Postconditioning on the Activation of Survival Kinases in Normal and Vascular Nitrate Tolerance States
To investigate the role of the activation of RISK pathway in IPost, we examined the activation of ERK 1/2 and PI3K-Akt pathways at the 10th minute of reperfusion in both nontolerant and nitrate tolerant rat hearts. The phos- phorylation of Akt on neither Ser308 nor Thr473 amino acid side chains showed significant difference between the groups (Fig. 3A, B). Similarly, the phosphorylation of eNOS, a down- stream target of Akt, did not show significant difference between the groups (Fig. 3C). Moreover, phosphorylation of neither 42 kDa nor 44 kDa isoforms of ERK showed significant difference between the groups (Fig 3D, E).
DISCUSSION
In the present study, we examined the effect of IPost on I/R injury and activation of survival kinases in the presence and absence of vascular nitrate tolerance. We have demonstrated here that the infarct size limiting effect of postconditioning was lost in the hearts of nitrate tolerant rats. Furthermore, we have shown that IPost did not lead to survival kinase activation in the rat myocardium and that the chronic treatment with nitroglyc- erin had no significant effect on the activation of RISK pathway. This is thefirst demonstration that nitrate pretreatment leading to the development of vascular nitrate tolerance abolishes the infarct size limiting effect of IPost.
It is a well-known phenomenon that brief periods of ischemia alternating with brief periods of reflow applied at the onset of reperfusion (ie, IPost) after a prolonged ischemic period reduces myocardial reperfusion injury in different species.26 However, recent focus of research has turned to investigate the effectiveness of IPost in the presence of risk factors or pathological states.1Iliodromitis et al27exam- ined the infarct size limiting effect of postconditioning in hypercholesterolemic and atherosclerotic rabbits. They found the abolishment of postconditioning in the presence of hyper- lipidemia. Similarly to these results, our research group observed the same phenomenon in cholesterol-fed rat hearts.9 Furthermore, the impairment of postconditioning was observed by Bouhidel et al.24 in obese ob/ob mice and by
FIGURE 3. The effect of nitrate tolerance and postcondition- ing on the phosphorylation state of Akt, eNOS, and ERK 1/2.
Diagrams show densitometric ratios of Akt phosphorylated on Thr308 (A) and Ser473 (B) amino acid side chains versus total Akt, phospho-eNOS/eNOS ratio (C), and phosphorylated and total ERK 1/2 (D and E) in each groups. Results are expressed as mean 6 standard error of mean (n = 5–8). Post, post- conditioning.
Przyklenk et al10 in diabetic murine models. These results show the impairment of myocardial stress adaptation in the presence of clinically relevant cardiovascular risk factors.
Apart from pathological states, chronic pharmacological treat- ments may also interfere with IPost. In a previous study, we have already demonstrated that chronic lovastatin treatment interferes with the infarct size limiting effect of postcondition- ing in isolated rat hearts.11
In our present study, we have shown for thefirst time in the literature that in the presence of vascular nitrate tolerance induced by repetitive injections of high-dose nitroglycerin, the cardioprotective effect of IPost was abolished. To date, there have been no studies investigating the interaction between IPost and nitrate treatment. However, a few studies looked at the interaction of preconditioning and nitrate treatment leading to the development of vascular nitrate tolerance. Szilvassy et al14have shown in a conscious rabbit model of precondition- ing that although the ST-segment elevation induced by rapid ventricular pacing was reduced by preconditioning, the cardi- oprotective effect of preconditioning was abolished in nitrate- tolerant animals. In contrast, in a different experimental model, Hill et al28proposed that long-term nitroglycerin treatment of rabbits leads to a decrease in myocardial infarct size when compared with nontolerant group. The effect of nitroglycerin treatment on the myocardial infarct size was similar to that of late preconditioning. However, in that study, 28-day-long transdermal nitroglycerin patch was used to induce vascular nitrate tolerance, and infarct size was measured 3 days after the patch removal, when the presence of vascular nitrate toler- ance is already questionable.
In the present study, we have also shown that the development of vascular nitrate tolerance did not affect infarct size significantly in hearts subjected to I/R. Conversely, Fan et al15have found that the presence of vascular nitrate tolerance induced by 12 hours of nitroglycerin infusion aggravated myo- cardial infarct size in rat hearts subjected to 40-minute coronary occlusion and 4-hour reperfusion in vivo. These discrepancies can be attributed to differences in nitrate tolerance induction and experimental design. In contrast to thesefindings, in a mul- ticenter human study, Ambrosio et al29 analyzed the data of more than 52,000 patients experiencing acute coronary syn- drome and found that nitrate use was associated with signifi- cantly lower cardiac markers of necrosis than nitrate-naive patients. Thisfinding indicated that chronic nitrate users tend to develop a smaller extent of myocardial necrosis.
Despite intensive research in the past decade, the molecular mechanism of postconditioning still remains to be elucidated. Several studies have suggested that the activation of RISK pathways (such as ERK 1/2 and PI3K-Akt pathways) detected at 5–15 minutes after reperfusion might play a crucial role in the cardioprotective effect of IPost.7,8,26In our present study, however, we have not found significantly increased Akt and Erk 1/2 phosphorylation by IPost in the risk area of the left ventricle 10 minutes after 30-minute ischemia neither in non- tolerant nor in nitrate tolerant rat hearts. As thefirst demonstra- tion of the possible involvement of PI3K-Akt signaling pathway in the mechanism of IPost, Tsang et al30described increased Akt phosphorylation in the ischemic myocardium at the seventh minute of reperfusion following 35 minute of regional
ischemia. The cardioprotection and Akt phosphorylation was diminished by pharmacologic inhibition of PI3K. Sim- ilar results were found by Bopassa et al31in ex vivo perfused rat hearts subjected to 30-minute global ischemia followed by reperfusion with or without postconditioning. However, they only examined the amount of P-Akt, but they did not measure P-Akt/Akt ratio, which gives more information about RISK pathway activation. Yang et al32also suggested the involvement of PI3K-Akt pathway in postconditioning based on measuring infarct size in the presence of the PI3K inhibitor wortmannin during postconditioning in ex vivo perfused rabbit hearts; however, they did not measure Akt phosphorylation. Furthermore, Darling et al33 showed that postconditioning increased the phospho-Erk 1/2 amount in the ischemic rabbit myocardium at thefifth minute of reper- fusion, but they did not find elevated Akt phosphorylation.
In contrast to thesefindings, recent studies suggest that post- conditioning does not activate RISK pathways in the early phase of reperfusion. Recently, Gonon et al34 have shown that combined treatment withL-arginine and IPost applied at the onset of reperfusion leading to cardioprotection does not lead either to Akt activation or to increased eNOS phosphor- ylation in the pig heart. Moreover, an article published by Skyschally et al35 described the reduction of infarct size by IPost without the activation of Akt or Erk 1/2 in an in vivo pig model. These data together with our present findings suggest that PI3K-Akt pathway does not play a crucial role in the molecular mechanism of postconditioning.
However, some other mechanisms apart from and in addition to the RISK pathway have been also proposed as alternative cardioprotective pathways. One of these alternative pathways potentially playing a role in IPost is the “survivor activating factor enhancement”pathway,36which is mediated by extracellular cytokine stress signals (interleukin-6 and tumor necrosis factor-alpha).26A major element of the survivor acti- vating factor enhancement pathway is the Janus kinase, which phosphorylates the transcription factor STAT3.26 It has been shown that pharmacological inhibition of the Janus kinase–
STAT pathway at the onset of myocardial reperfusion in ex vivo Langendorff perfused mice hearts,37or its genetic ablation in mice,38abolished the infarct size limiting effect of postcon- ditioning. Other possible alternative mechanisms potentially playing a role in IPost are activation of sphingosine kinase,39 protein kinase C,40and NO-soluble guanylate cyclase-cGMP- protein kinase G–mediated pathway,41 postconditioning- induced early increase in peroxynitrite formation,9or inhibition of matrix metalloproteinases.42 Many of the aforementioned potentially protective mechanisms have been proposed to atten- uate the opening of the mitochondrial permeability transition pore.26Although multiple signaling pathways might be altered by IPost, the relative significance of these pathways is to be elucidated.
To date, there were no studies investigating the effect of vascular nitrate tolerance on myocardial survival kinase activation. In our present study, however, we have not found any significant effect of nitrate treatment on the activation of RISK pathway. Other possible mechanisms responsible for the loss of postconditioning in vascular nitrate tolerance remain to be investigated in further studies.
CONCLUSIONS
In conclusion, this is the first demonstration that nitrate treatment leading to the development of vascular nitrate tolerance interferes with the infarct size limiting effect of IPost.
Furthermore, we have also shown that survival kinase activation does not play a crucial role in the mechanism of IPost.
REFERENCES
1. Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning.Pharmacol Rev.2007;59:418–458.
2. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia:
a delay of lethal cell injury in ischemic myocardium. Circulation.
1986;74:1124–1136.
3. Granfeldt A, Lefer DJ, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning.Cardiovasc Res.2009;83:
234–246.
4. Peart JN, Headrick JP. Clinical cardioprotection and the value of condition- ing responses.Am J Physiol Heart Circ Physiol.2009;296:H1705–H1720.
5. Skyschally A, van Caster P, Iliodromitis EK, et al. Ischemic postcondi- tioning: experimental models and protocol algorithms.Basic Res Cardiol.
2009;104:469–483.
6. Balakumar P, Rohilla A, Singh M. Pre-conditioning and postcondition- ing to limit ischemia-reperfusion-induced myocardial injury: what could be the next footstep?Pharmacol Res.2008;57:403–412.
7. Hausenloy DJ. Signalling pathways in ischaemic postconditioning.
Thromb Haemost.2009;101:626–634.
8. Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning.Cardiovasc Res.2006;70:240–253.
9. Kupai K, Csonka C, Fekete V, et al. Cholesterol diet-induced hyperlipid- emia impairs the cardioprotective effect of postconditioning: role of per- oxynitrite.Am J Physiol Heart Circ Physiol.2009;297:H1729–H1735.
10. Przyklenk K, Maynard M, Greiner DL, et al. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type- 1 diabetes.Antioxid Redox Signal.2011;14:781–790.
11. Kocsis GF, Pipis J, Fekete V, et al. Lovastatin interferes with the infarct size-limiting effect of ischemic preconditioning and postconditioning in rat hearts.Am J Physiol Heart Circ Physiol.2008;294:H2406–H2409.
12. Csont T, Ferdinandy P. Cardioprotective effects of glyceryl trinitrate:
beyond vascular nitrate tolerance.Pharmacol Ther.2005;105:57–68.
13. Nossaman VE, Nossaman BD, Kadowitz PJ. Nitrates and nitrites in the treatment of ischemic cardiac disease.Cardiol Rev.2010;18:190–197.
14. Szilvassy Z, Ferdinandy P, Nagy I, et al. The effect of continuous versus intermittent treatment with transdermal nitroglycerin on pacing-induced preconditioning in conscious rabbits.Br J Pharmacol.1997;121:491–496.
15. Fan Q, Gao F, Zhang L, et al. Nitrate tolerance aggravates postischemic myocardial apoptosis and impairs cardiac functional recovery after ischemia.
Apoptosis. 2005;10:1235–1242.
16. Gori T, Dragoni S, Di Stolfo G, et al. Tolerance to nitroglycerin-induced preconditioning of the endothelium: a human in vivo study.Am J Physiol Heart Circ Physiol.2010;298:H340–H345.
17. Csont T. Nitroglycerin-induced preconditioning: interaction with nitrate tolerance.Am J Physiol Heart Circ Physiol.2010;298:H308–H309.
18. Csont T, Csonka C, Onody A, et al. Nitrate tolerance does not increase production of peroxynitrite in the heart.Am J Physiol Heart Circ Physiol.
2002;283:H69–H76.
19. Csont T, Pali T, Szilvassy Z, et al. Lack of correlation between myocar- dial nitric oxide and cyclic guanosine monophosphate content in both nitrate-tolerant and -nontolerant rats. Biochem Pharmacol. 1998;56:
1139–1144.
20. Csont T, Szilvassy Z, Fulop F, et al. Direct myocardial anti-ischaemic effect of GTN in both nitrate-tolerant and nontolerant rats: a cyclic GMP- independent activation of KATP.Br J Pharmacol.1999;128:1427–1434.
21. Bencsik P, Kupai K, Giricz Z, et al. Role of iNOS and peroxynitrite- matrix metalloproteinase-2 signaling in myocardial late preconditioning in rats.Am J Physiol Heart Circ Physiol.2010;299:H512–H518.
22. Turan N, Csonka C, Csont T, et al. The role of peroxynitrite in chemical preconditioning with 3-nitropropionic acid in rat hearts.Cardiovasc Res.
2006;70:384–390.
23. Csonka C, Kupai K, Kocsis GF, et al. Measurement of myocardial infarct size in preclinical studies.J Pharmacol Toxicol Methods.2010;61:163–170.
24. Bouhidel O, Pons S, Souktani R, et al. Myocardial ischemic postcondi- tioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol.2008;295:H1580–H1586.
25. Gorbe A, Giricz Z, Szunyog A, et al. Role of cGMP-PKG signaling in the protection of neonatal rat cardiac myocytes subjected to simulated ischemia/reoxygenation.Basic Res Cardiol.2010;105:643–650.
26. Ovize M, Baxter GF, Di Lisa F, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology,Cardiovasc Res.2010;87:406–423.
27. Iliodromitis EK, Zoga A, Vrettou A, et al. The effectiveness of postcon- ditioning and preconditioning on infarct size in hypercholesterolemic and normal anesthetized rabbits.Atherosclerosis. 2006;188:356–362.
28. Hill M, Takano H, Tang XL, et al. Nitroglycerin induces late precondi- tioning against myocardial infarction in conscious rabbits despite devel- opment of nitrate tolerance.Circulation. 2001;104:694–699.
29. Ambrosio G, Del Pinto M, Tritto I, et al; GRACE Investigators. Chronic nitrate therapy is associated with different presentation and evolution of acute coronary syndromes: insights from 52,693 patients in the Global Registry of Acute Coronary Events.Eur Heart J.2010;31:430–438.
30. Tsang A, Hausenloy DJ, Mocanu MM, et al. Postconditioning: a form of
"modified reperfusion" protects the myocardium by activating the phos- phatidylinositol 3-kinase-Akt pathway.Circ Res.2004;95:230–232.
31. Bopassa JC, Ferrera R, Gateau-Roesch O, et al. PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postcondi- tioning.Cardiovasc Res.2006;69:178–185.
32. Yang XM, Philipp S, Downey JM, et al. Postconditioning’s protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation.Basic Res Cardiol.2005;100:57–63.
33. Darling CE, Jiang R, Maynard M, et al. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/
2.Am J Physiol Heart Circ Physiol.2005;289:H1618–H1626.
34. Gonon AT, Jung C, Yang J, et al. The combination of L-arginine and ischemic postconditioning at the onset of reperfusion limits myocardial injury in the pig.Acta Physiol (Oxf).2011;201:219–226.
35. Skyschally A, van Caster P, Boengler K, et al. Ischemic postconditioning in pigs: no causal role for RISK activation.Circ Res.2009;104:15–18.
36. Lecour S. Activation of the protective survivor activating factor enhance- ment (SAFE) pathway against reperfusion injury: does it go beyond the RISK path?J Mol Cell Cardiol.2009;47:32–40.
37. Goodman MD, Koch SE, Fuller-Bicer GA, et al. Regulating RISK: a role for JAK-STAT signaling in postconditioning?Am J Physiol Heart Circ Physiol.2008;295:H1649–H1656.
38. Boengler K, Buechert A, Heinen Y, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice.Circ Res.
2008;102:131–135.
39. Thielmann M, Dörge H, Martin C, et al. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha and sphingosine. Circ Res.
2002;90:807–813.
40. Zatta AJ, Kin H, Lee G, et al. Infarct-sparing effect of myocardial post- conditioning is dependent on protein kinase C signalling.Cardiovasc Res.2006;70:315–324.
41. Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia/reperfusion: opportunities and obstacles for survival signaling.Br J Pharmacol.2007;152:855–869.
42. Romero-Perez D, Fricovsky E, Yamasaki KG, et al. Cardiac uptake of minocycline and mechanisms for in vivo cardioprotection.J Am Coll Cardiol.2008;52:1086–1094.