CHAPTER I X
Assay of Ovarian Hormones B Y G R E G O R Y P I N C U S
CONTENTS
Page
Introduction 333 International Standards 334
I. Chemical Methods of Assay 334
A. Estrogens 334 B. Progesterone 335 II. Physical Methods of Assay 340
A. Estrogens—Absorption Spectrophotometry 340 B. Progesterone—Absorption Spectrophotometry 340
C. Estrogens—Polarographic Assay 340
III. Biological Assay Methods 341 A. Estrogens—Vaginal Smear Methods 341
1. Modified Butenandt Procedure 341 2. Mather Modification of Marrian-Parkes Procedure 343
3. Thayer-Doisy Procedure 343 4. Allen-Doisy Procedure 344 5. Curtis-Doisy Procedure 344 B. Estrogens—Other Methods 345
C. Progesterone 346 References 347
Introduction
The numerous methods that have been used for the quantitative determination of biologically active substances may be divided into:
chemical (I), physical (II), and biological (III). Measurement of quan- tities and concentrations of the estrogens and of progesterone have been made by all three methods. It is not possible to present all of the pro- posed techniques in detail. Accordingly those which are practical for usual assay purposes will be explicitly presented and reference will be made to others where requisite. In general assay methods have been employed for two purposes: the determination of active hormones as chemicals or pharmaceuticals, and the determination of active hormones in body tissues and fluids. In the former case macromethods are often applicable and desirable, in the latter case micromethods are ordinarily mandatory because of the minute or at best rather small concentration of these hormones in the body and its excreta.
333
334 GREGORY P I N C U S
International Standards
Two estrogenic substances have been set up as International Stand- ards under League of Nations conferences (69,70) on hormone standardi- zation. Crystalline estrone was contributed to the estrone reference standard and the international unit was defined as the specific estrus- producing activity contained in 0.1 μg. The international unit of estradiol benzoate is 0:1 /zg. of this compound. The international unit of progesterone is the specific progestational activity contained in 1 mg.
of crystalline hormone.
I. Chemical Methods of Assay A . ESTROGENS
The most obvious and ultimately the definitive method of chemical measurement of presumed estrogens is the weighing of the isolated crystal- line compounds and their identification by melting points, melting points of derivatives, and other physical characteristics. This involves methods of fractionation and purification given in the next chapter, which need not concern us here. The gravimetric determination of estriol in late human pregnancy urine and of estrone in mare's pregnancy urine is practicable in view of the relatively large amounts obtainable and the possibility of separating these compounds out almost quantitatively by fairly simple fractionation and subsequent chromatography. In all other instances in which analysis of animal estrogens has been sought, isolation is not practicable unless extremely large quantities of starting material are used and ordinarily rather complex extraction procedures employed. In the practice of either clinical analyses or research investi- gations the most common microchemical method of determination employed is an estrogen color reaction.
Kober (45) was the first to propose a color reaction for urinary estro- gen; he observed that phenolsulfonic acid reacted with estrone and urinary extracts to give a pink color. Since Kober's publication various investigators have studied one or more modifications of his reaction and examined other colorimetric reactions of estrogens. The pertinent publications are summarized in Table I. It should be pointed out that in most mammalian tissues and fluids the naturally occurring estrogens are estrone, estradiol (ß-estradiol in the rabbit, α-estradiol in all other mammals examined, see Pincus and Pearlman, 59, and Doisy, 25), and estriol. Equilin, equilenin, and related estrogens appear to be peculiar to the horse family. Ideally one should have a specific color reaction for each of these that may be developed quantitatively in the presence of other compounds present in the extractions containing the estrogens.
IX. ASSAY OF OVARIAN HORMONES 335 This ideal has not been met. In every procedure proposed in Table I a greater or lesser amount of purification of the original sources of the estrogens is required. In none of the purification procedures employed is an absolute quantitative yield of all of the estrogens assured. Further- more in most instances accompanying estrogens in the final extracts are nonestrogenic chromogens which react with the reagents employed to give either the same or some other color. It appears probable that the urines of late pregnancy may be assayed colorimetrically for their estro- gen content. By all methods, except possibly that of Stimmel, the estrogen content of nonpregnancy or early pregnancy urines is not ascertainable due to the presence of interfering chromogens. Even in StimmeFs method the use of a color correction equation is requisite (78), and then can apparently be applied safely only to the midmenstrual peak of excretion in women. Finally none of the methods appear to be applicable to extracts of blood, body tissues, feces, or the urines of men.
B. PROGESTERONE
The gravimetric determination of pure progesterone as isolated from corpus luteum tissue or whole ovaries is the only quantitative method known. No practicable color reactions have been proposed. The almost complete absence of progesterone from urine has not encouraged the sort of development observed in estrogen microcolorimetry.
The chief metabolite of progesterone is pregnanediol. It has been measured quantitatively in pregnancy urine and in the urines of the luteal phase of the menstrual cycle as pregnanediol glucuronide by the method of Venning (87). "Spontaneous'' hydrolysis of the glucuronide must be guarded against by preserving the urine with sodium cyanide.
Colorimetric measurement of the glucuronic acid freed on hydrolysis has been advocated to avoid certain steps in the Venning procedure (42).
Where sufficient quantities of pregnanediol are present, direct isolation is advocated (Astwood and Jones, 5). The method of Astwood and Jones gives values 20% to 40% lower than those obtained with the Venning method (Bachman, 8). Marrian's recent finding (51) that glucuronides obtained by the Venning method contain considerable amounts of the pregnanolones may account for this difference. A color reaction of pregnanediol with sulfuric acid (82) can be applied only if the free pregnanediol is relatively uncontaminated by impurities. Its appli- cation to the neutral, alcoholic, nonketonic fraction of human urine has therefore been suggested (56).
The pregnanolones are 20-ketosteroids found in human pregnancy urines. Although chemically quite similar to pregnanediol, they prob-
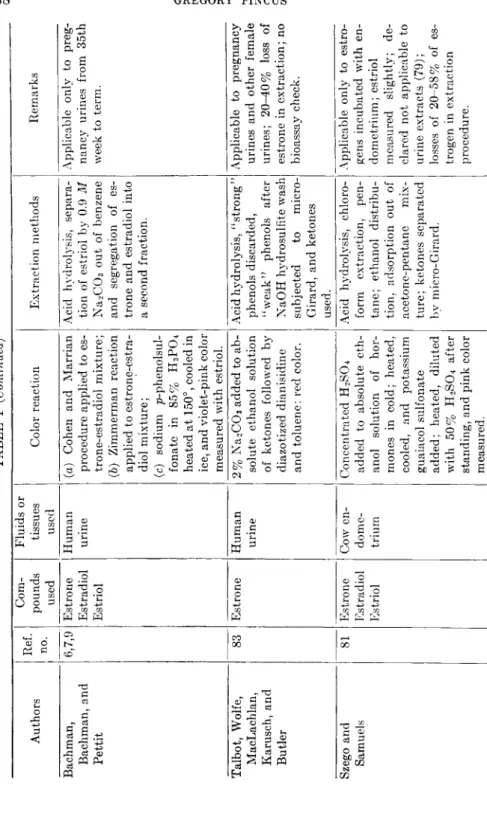
TABLE I COLORIMETRIC METHODS FOR THE ASSAY OF ESTROGENS Authors Ref. no.
Com- pounds used
Fluids or tissues used Color reaction Extraction methods Remarks Cohen and Marrian 16 Estrone Estriol Human urine Heat with phenolsulfonic acid, cool in ice bath and dilute with 5% H2S04, cool and measure pink color.
Acid hydrolysis, separa- tion of "weak" phenols from "strong" phenols.
Applicable only to late pregnancy urines; non- estrogenic chromogen contribution probable; estrone and estradiol in same fraction. David 23 Estriol None Heat with concentrated H2S04, dilute and add arsenic acid; measure blue color.
None. Specific for estriol. Sola 76 Estrone Mare's pregnancy urine
Heat with concentrated H2SO4, and measure greenish fluorescence.
Acid hydrolysis, separa- tion of urinary phenols. Qualitative test only. Zimmerman 93 Estrone None Add alcoholic ra-dinitro- benzene and concen- trated KOH solution, and measure pink color.
None. All ketones react with this reagent but 17- ketones give character- istic pink color. Cartland, Meyer, Miller, and Rutz 13 Estrone Stallion urine Heat with phenolsulfonic and cool; dilute with H20, heat, cool, dilute with H20, and measure pink color.
Butanol extraction, acids removed, and Buten- andt (11) partition fol- lowed.
"Cloudy" solutions often develop with crude urine extracts. Schmulovitz and Wylie 74 Estrone (theelin) Human pregnancy urine
Diazotized p-nitroaniline added to alcoholic NaCC>3solution, "wine" color measured.
Urine concentrated at pH 4, extracted with ethyl ether; washed with NaC03, ether and vola- tile phenols distilled off.
Used for pregnancy diag- nosis.
Pincus, Wheeler, Young, and Zahl 63 Estrone Estradiol Estriol
Human urine, rabbit urine
(a) Cohen and Marrian procedures; (b) procedures of Cart- land et al.; (c) David procedure; (d) to hormone in chloro- form solution add ZnCU solution followed by ben- zoyl chloride, heat, cool, dilute with chloroform, and measure color.
Acid hydrolysis, separa- tion of "weak" phenols from "strong" phenols.
(a) Applicable only to late pregnancy urines, better than (b) for these; both react with non- estrogenic chromogens in rabbit urines. (c) specific but not appli- cable to urine extracts as purified. (d) estriol does not react; checks with (a) in late pregnancy urines; gives intense reaction with nonestrogenic chromo- gens in rabbit urines. Pincus and Zahl 64 Estrone Estradiol Estriol
Rabbit urine Sulfanilic acid and NaN02 in aqueous solution added to alcoholic hor- mone solution, coupling on adding NaOH solu- tion; measure red color.
Acid hydrolysis, separa- tion of "weak" phenols from "strong" phenols.
Applicable only to unpig- mented extracts; less sensitive than Kober re- action; estrone and es- tradiol in same fraction. Venning, Evelyn, Harkness, and Browne
89 Estrone Estradiol Estriol
Human urine Phenolsulfonic acid added to dry extract, heated 20 min., diluted with H20 after cooling, re- heated, cooled and 10% H2S04 added. Pink color developed.
Butanol extraction of acidified urine, acid hy- drolysis of butanol ex- tract and ether extract of hydrolyzate.
Applicable to human pregnancy urines con- taining 500 Mg. or more of estrogen; color cor- rection for nonestrogenic chromogen necessary; some inhibition of color development by preg- nandiol.
TABLE I (Continued) Authors Ref. no.
Com- pounds used
Fluids or tissues used Color reaction Extraction methods Remarks Bachman, Bachman, and Pettit
6,7,9 Estrone Estradiol Estriol
Human urine (a) Cohen and Marrian procedure applied to es- trone-estradiol mixture; (b) Zimmerman reaction applied to estrone-estra- diol mixture; (c) sodium p-phenolsul- fonate in 85% H3P04 heated at 150°, cooled in ice, and violet-pink color measured with estriol.
Acid hydrolysis, separa- tion of estriol by 0.9 M Na2C03 out of benzene and segregation of es- trone and estradiol into a second fraction.
Applicable only to preg- nancy urines from 35th week to term. Talbot, Wolfe, MacLachlan, Karusch, and Butler
83 Estrone Human urine 2% Na2C03 added to ab- solute ethanol solution of ketones followed by diazotized dianisidine and toluene; red color.
Acid hydrolysis, "strong" phenols discarded, "weak" phenols after NaOH hydrosulfite wash subjected to micro- Girard, and ketones used.
Applicable to pregnancy urines and other female urines; 20-40% loss of estrone in extraction; no bioassay check. Szego and Samuels 81 Estrone Estradiol Estriol
Cow en- dome- trium
Concentrated H2SO4 added to absolute eth- anol solution of hor- mones in cold; heated, cooled, and potassium guaiacol sulfonate added; heated, diluted with 50% H2SO4 after standing, and pink color measured.
Acid hydrolysis, chloro- form extraction, pen- tane; ethanol distribu- tion, adsorption out of acetone-pentane mix- ture; ketones separated by micro-Girard.
Applicable only to estro- gens incubated with en- dometrium; estriol measured slightly; de- clared not applicable to urine extracts (79) ; losses of 20-58% of es- trogen in extraction procedure.
Jayle, Crepy, and Judas 41 Estrone Human urine Kober reaction with ace- tone added, which pre- serves nonestrogenic colors overnight while pink color fades.
Acid hydrolysis, ether ex- traction, " estriol" taken out of benzene solution of ether extract and estrone-estradiol mixture left.
Applicable to human pregnancy urine con- taining 500 jug. or more of estrogen. Find en- zymic hydrolysis of estrogens useless. Reifenstein and Dempsey 68 Estrone Human urine Procedure of Talbot et al. Acid hydrolysis, "strong" phenol removed, "weak" phenols sub- jected to micro-Girard separation and ketone fraction assayed.
Apparently overestimates estrone due to nonestro- genic chromogens, 27- 54% of estrone lost in extraction. Veitch and Milone 86 Estrone None 2,4-dinitrophenylhydra- zone of estrone added to 0.1 Ν alcoholic KOH and deep red color meas- ured.
Chromatography of hy- drazone suggested. Applicability to tissues or fluids not known. Stimmel 77 78 Estrone Estradiol Estriol
Human urine Kober reaction to "es- tradiol" fraction, Zim- merman (93) reaction to estrone fraction and Bachman (9) reaction to estriol fraction.
Butanol extract acid hy- drolyzed, acids removed with carbonate and chro- matography of ether concentrate out of ben- zene.
Applicable to pregnancy urines from 24th week on and to midmenstrual rise with color correc- tion. CO CO SC
340 GREGORY PINCUS
ably do not represent metabolites of progesterone (59), but are more likely metabolites of adrenal steroid precursors (88).
II. Physical Methods of Assay
A . ESTROGENS—ABSORPTION SPECTROPHOTOMETRY
The natural estrogens show a characteristic absorption spectrum in the ultraviolet due to the presence of the phenolic ring. The absorption maximum is at 280 πΐμ in ethanol solution. The equilenin series differs from the estrone series because of the naphthalenic structure of the former (43). Chevallier et al. (14) have used this absorption peak as a qualitative test for estrone in mare's pregnancy urine, but in most urines there are interfering substances in crude fractions. Friedgood and Garst (32) have recently re-examined the problem of the conditions necessary for the separation of mixtures of pure estrone, estradiol, and estriol by methods which entail minimal losses and typical ultraviolet absorption curves. A procedure involving 48 extraction steps was evolved, but it has not been applied to biological specimens.
The infrared spectrum of the estrogens has been examined with a view to their assay by this method (33). The estrogens are distinguished by four bands: at 11.30 to 11.42, 8.60 to 8.70, 6.20 to 6.30, and 6.65 to 6.70μ. Thus far no application to biological specimens has been reported.
B. PROGESTERONE—ABSORPTION SPECTROPHOTOMETRY
The conjugated ketone of ring A of progesterone shows a typical ultraviolet absorption maximum at 240 πΐμ (in ethanol). This type of absorption is exhibited by the other steroid hormones having this α,β- unsaturated ketone structure (see 43). Reynolds and Ginsburg (71) have made an approach to its utilization as a means of detecting proges- terone in blood. Its practical application has, however, not been realized as yet. The conjugated 3-carbonyl in steroids is associated also with an absorption maximum in the infrared at 1678 c m .- 1 (43), but specific recognition of progesterone in the presence of other absorbing compounds has not been achieved.
C. ESTROGENS—POLAROGRAPHIC ASSAY
Estrone couples with the ketone reagent of Girard to give a water- soluble compound having a specific current discharge on reduction at the dropping mercury electrode measurable polarographically (92). By appropriate adjustment microquantities of this ketone complex may be measured (10,90). The neutral 17-ketosteroids of human urine have been measured by this method (90,92), and the measurement of estrone is also being attempted (91).
IX. ASSAY OF OVARIAN HORMONES 341 III. Biological Assay Methods
A. ESTROGENS—VAGINAL-SMEAR METHODS
Standard estrogen bioassays are based on the Allen-Doisy vaginal- smear method (2,44). It has been modified considerably by numerous investigators in order to increase its sensitivity and reliability. In each instance the essential procedure is the administration of standard estro- gen to ovariectomized rats or mice and the examination of the vaginal smear at a given interval or various intervals after estrogen administra- tion for the cornified epithelial cells that arise as the result of estrogen action in the otherwise quiescent vaginal epithelium. On the basis of the percentage of animals showing cornified (positive) smears with increasing estrogen dosage a dosage-response curve is constructed. Ordinarily the dosage which results in positive smears in 50% of the animals is desig- nated as the unit of estrogen. A considerable number of factors will determine the amount of estrogen necessary for a unit dose. The role of various of these factors has been reviewed in a number of critical publications (18,22,24,29,44,52,55,67). Tables II and III illustrate most of the contributing variables.
Table II is taken from the data of Pedersen-Bjergaard (55). It demonstrates that identical methods of administration to different species lead to quite different assay units and that the ratio of activities between two estrogens varies from species to species. The solvent employed and the frequency and route of administration all affect the derived assay unit. Although taking the activity per weight of animal tends to reduce the marked difference in unit size from species to species (e.g., the sub- cutaneous oily injections), wide variations are still encountered.
Table III is taken from a paper by Thayer, Doisy, and Doisy (84).
In testing the comparative activities of estrone β- and α-estradiol and
^-estradiol benzoate, five bioassay methods were employed. These were as follows:
1. Modified Butenandt Procedure
Single Injection of Estrogen in Oil, Mice (12). "Mice were ovariec- tomized as previously described by Allen and Doisy (1). Vaginal smears were made daily for a period of two weeks following ovariectomy and animals that did not show a negative smear each day were discarded.
Two weeks after ovariectomy, the mice were primed with an aqueous solution of estrone containing 0.1 y or one international unit. Smears were taken at 9:00 a.m., 1:00 p.m., and 5:00 p.m., starting 48 hours after the injection.
342 GREGORY PINCUS T A B L E I I
E F F E C T OF M E T H O D S OF A D M I N I S T R A T I O N A N D S P E C I E S OF T E S T O V A R I E C T O M I Z E D A N I M A L S ON T H E E S T R O G E N I C B I O A S S A Y OF T H E T W O I N T E R N A T I O N A L
S T A N D A R D P R E P A R A T I O N S
Animal Solvent Route"
No. of admin- istra- tions
Estrone Estradiol benzoate Relative potency, unit of estrone/
unit of estradiol benzoate Animal Solvent Route"
No. of admin- istra- tions
Assay unit, MG.
U n i t / 20 g.
animal, MG-
Assay unit,
MG-
Unit/
20 g.
animal, MG-
Relative potency, unit of estrone/
unit of estradiol benzoate
Mouse Oil S 1 0.35 0 . 3 5 0.108 0.108 3.20 Water CO 5 0.19 0.19 0 . 2 2 0 . 2 2 0.86 Water 0 5 11.70 11.70 11.50 11.50 1.00 Rat Oil CO 1 2 . 7 6 0.28 0.28 0.03 9.90 Water S 5 1.11 0.11 0.28 0.03 4 . 0 0 Water 0 5 220.00 2 2 . 0 0 225.00 2 2 . 5 0 0.98 Guinea Oil CO 1 600.00 15.00 4 5 . 0 0 1.13 13.00 PIG Water CO 5 460.00 11.50 3 0 . 0 0 0.75 15.00
Water 0 5 8900.00 223.00 b
Monkey Oil S 1 1000.00 5.00 2 5 . 0 0 0.13 4 0 . 0 0 Water 0 1 5000.00 2 5 . 0 0 100,000.00 500.00 0.05
a S = subcutaneous; Ο = oral.
b Ineffective at any level tested.
T A B L E I I I
P O T E N C I E S OF ^ - E S T R A D I O L , /^-ESTRADIOL M O N O B E N Z O A T E , « - E S T R A D I O L , AND E S T R O N E AS D E T E R M I N E D BY F I V E D I F F E R E N T M E T H O D S OF B I O A S S A Y
Compounds"
Butenandt modified units/mg.
mice
Marrian- Parkes modified units/mg.
mice
Thayer- Doisy units/mg.
mice
Allen- D o isy modified units/mg.
rats
Curtis- Doisy modified units/mg.
rats
/3-Estradiol 700 8000 2000 200 700
«-Estradiol 35,000 70,000 40,000 17,000 5000 Estrone 20,000 35,000 20,000 1700 1000 ß-Estradiol benzoate. . . . 1000 2400 450 200 200
ff1 28 4 . 4 10 8.5 1.4
R2 1.4 0 . 3 0.22 1. 0 . 3
Potency of estrone ^2 _ Potency of ff-estradiol-3-beiizoate Potency of ß-estradiol' ~~ Potency of ß-estradiol
IX. ASSAY OF OVARIAN HORMONES 343
"One week after priming the mice were divided into two groups of 20 animals. Each of one group of 20 animals was injected subcutan- eously at 9:00 a.m. with the oily preparation being tested; each of a similar group was injected with the standard estrone preparation dis- solved in oil, and the proportion of positive effects in each group was determined. After one or two trial experiments, the dosage could usually be so adjusted that approximately the same percentage responses to the unknown and standard were obtained. In accordance with the work of Coward and Burns (18) a 50 per cent positive response was regarded as one unit; the unknown was evaluated in terms of the standard response curve and the concurrent response to the standard.
"Mice that failed to respond positively to an injection within 80 hours were immediately primed with an oily solution containing 0.05 γ of estrone. Owing to the slow rate of absorption mice were not used again until four weeks had elapsed following a positive response. The volume of oil injected was usually 0.2 cc. and in all experiments the unknown and standard were administered in the same volume.''
2. Mather Modification of the Marrian-Parkes Procedure
Four Injections of an Aqueous Solution, Mice {52,53). "Ovariec- tomized mice were given subcutaneous injections of four equal quantities of an aqueous solution at 8:00 a.m. and 5:00 p.m., starting 16 hours after the last injection, and the last smear was taken 48 hours after the first smear. In this method the same principles were followed as in the modified Butenandt method, using comparable animals and the same method of determining potency of a preparation. The estrogens and the standard preparation used in this assay procedure were dissolved in an aqueous medium. Animals that showed a negative response from previous injections were primed with an aqueous solution containing 0.1 γ of estrone before they were used for another assay. In this method the test animals were used every two weeks/'
3. Thayer-Doisy Procedure
Three Injections of an Aqueous Solution, Mice. "Injections were made at 9:00 a.m., 1:00 p.m., and 5:00 p.m. on the same day. The same principles regarding priming, estimation of activity, etc. which were used in the preceding method were followed in this procedure, with the exception that the mice were used for assay at intervals of seven days. We have used this method for many years as a routine procedure to determine the biological activity of estrogens."
344 GREGORY PINCUS
4. Allen-Doisy Procedure
Three Injections of an Aqueous Solution, Rats (1). "Ovariectomized rats were given subcutaneous injections of three equal quantities of an aqueous solution at intervals of four and one-half hours. All rats used had given a positive response one week before use for assay due to priming with 1.2 γ of estrone or a previous assay. The same principles were fol- lowed as in the other procedures for determination of units. In this method the changes of the cells in the vagina may be regarded as positive if a few leukocytes are present along with the nucleated epithelial cells and squamous nonnucleated epithelial cells."
5. Curtis-Doisy Procedure
Six Injections of Aqueous Solution, Immature Rats (19). "The estro- gen was injected at 9:00 a.m. and 5:00 p.m. on three successive days.
Changes in the procedure from the original method as published by Curtis and Doisy were: the requirement of opening of the vagina with a positive smear, and the application of the same procedure for determina- tion of units as in the other assay methods."
These represent current and frequently employed methods of bio- assay. It may be seen that the latent period between ovariectomy, priming, and successive assays give marked differences in sensitivity (see methods 1, 2, and 3) as does the criterion of a positive response. It should be noted that ß-estradiol benzoate does not have the same potency as the free estrogen so that not only are there differences between the different estrogens but between derivatives of the same estrogen.
There are other variables to be considered. These are: (a) the diet of the animals, e.g., vitamin A deficiency leads to vaginal cornification (31) and certain Β complex deficiencies to absence of estrogen effect (35);
(b) the method of taking vaginal smears (e.g., too frequent smearing per se leads to vaginal cornification, 44) ; (c) seasonal or climatic changes that lead to significant fluctuations in the response of test animals (21,29,54); and (d) the peculiar insensitivity to estriol that develops on its repeated administration to the same animal (20).
It is clear from the foregoing that standard preparations of pure estrogen should be characterized by the name and specific physical and chemical constants of the contained substance. If biological units are stated the exact method of assay should be given. The United States Pharmacopeia in setting up reference standards for the individual estro- gens is attempting to formulate a useful standard bioassay procedure.
An approved method, based on the use of spayed animals, should appear in the thirteenth edition of the U.S.P.
IX. ASSAY OF OVARIAN HORMONES 345
B . ESTROGENS—OTHER METHODS
Other types of test animals and criteria of activity have been sug- gested as useful in estrogen assay. The increase in uterine weight effected by estrogens in immature animals offers the opportunity for a graded response method. Astwood (3) has suggested utilizing the increase in uterine water observed in rats at about six hours after estradiol administration. It has the advantage of great sensitivity, but the response to various estrogens is quantitatively different (62). The uterine hypertrophy occurring after several days administration may also be standardized (26,27,46), but an extensive characterization of controlling conditions has not been presented. Extreme sensitivity is encountered when estrogen is applied intravaginally in castrated females (30,48,65), but again data sufficiently exact for standard conditions or quantification are lacking. Recently an extremely sensitive response to estrogen, the disappearance of the vaginal closure membrane of the guinea pig, has been described (34). As little as 0.0008 Mg. of estradiol diproprionate gives a positive response; great variability, however, seems to characterize the effect (47).
In dealing with the bioassay of impure preparations a further com- plication is added to the variables encountered in the assay of crystalline estrogens. In urine extracts for example, there occur unknown sub- stances that may either augment or inhibit the activity of the contained estrogens (28,57). For such preparations, therefore, even an approxi- mately accurate estimation of contained estrogens may be had only by purification and separation of fractions containing each individual estrogen. Methods for such fractionation on a micro scale have been developed by several investigators (56-58,75), but they have been applied chiefly to urine. Applicability to estrogen-enriched serum perfusates has been indicated (72,73). The recent finding that blood estrogens are bound to protein (80) indicates the need for further refinement of methods in dealing with the naturally occurring compounds in this fluid and prob- ably also in various organs and tissues.
If bioassay of estrogens is to be practised with quantitative accuracy and reliability, it is clear that certain desiderata must be met. The test animal should respond with minimum variability to the standard estro- gen in easily repeatable fashion; the preparation to be tested must be sufficiently pure to represent the specific standard estrogen for which assay is sought. The bioassay procedure for estrone in spayed rats pre- sented by Curtis et al. (21) appears to be very nearly the one of choice for estrone; a unit of 1.1 Mg. of estrone is repetitively encountered with an
accuracy of ± 1 5 % (99 times out of 100) using fifteen to twenty animals
346 GREGORY PINCUS
for each of three dosage levels. Similar accuracy by a roughly similar method is obtained with estradiol and estriol (57). For greater sensi- tivity a completely reliable method is yet to be developed. In any method employed the limits of accuracy must be strictly defined. The necessary statistical methods have been excellently presented by Emmens (29), Curtis et al. (21), and Pugsley (66). The bald statement of so many rat units or mouse units in a preparation is inadequate unless the limits of variation, the specific test, and the specific estrogen are included.
C. PROGESTERONE
The progestational proliferation of the uterine endometrium of the rabbit in response to progesterone administration has been the basis of the chief methods of bioassay for this hormone. The original method of Corner and Allen (17) requires the use of adult female rabbits ovariec- tomized at eighteen hours after a fertile mating. A sample portion of the uterine horn removed at ovariectomy is sectioned and compared micro- scopically with a comparable portion removed one day after the last of five daily subcutaneous injections of progesterone in oil. The degree of pseudopregnant proliferation in the latter sections is graded on a scale extending from — to + + + + . The assay unit is set at a level given by
+ + + , and in numerous assays this response has been obtained gen- erally with one milligram of crystalline progesterone. Increased sensi- tivity is had by the use of immature female rabbits primed with estrogen and then injected with progesterone (15,50), so that a unit dose is then about 0.6 to 0.7 mg. The presence of estrogen in extracts to be assayed may vitiate these tests, and no statistical analysis setting the limits of accuracy is available.
Pincus and Werthessen (60) have devised methods of measuring the endometrial response in mature female rabbits and have included meas- urements of the ovum growth response to progesterone. A dosage of 0.38 mg. progesterone may be detected in a single animal (98 times out of 100). Absolute accuracies may also be given on the basis of the num- ber of animals employed for assay.
The intrauterine application of progesterone to the rabbit permits the detection of small amounts of progesterone. McGinty et al. (49) were able to detect an effect of 0.13 μg. in the primed immature rabbit.
Hoskins (40) extended their observations and attempted quantification on the basis of the uterine mitoses resulting from progesterone stimulation.
Although he could measure 0.06 to 1.7 Mg. by this method, the variability was considerable. The intrauterine method has been used to measure blood progesterone by Hoffmann and von Lam (37).
The contraction after adrenaline administration of the uterus of the
IX. ASSAY OF OVARIAN HORMONES 347 progesterone-treated cat is the basis for an assay proposed by Van Dyke and Chen (85). A good dosage-response curve is obtained with a unit of about 0.45 mg. (the dosage per cat necessary to produce the uterine con- traction in 50% of the animals).
Astwood (4) obtains a similar unit in measuring the minimum dosage required to elicit a deciduomatous response in the spayed pseudopregnant rat.
The copulatory reflex in the spayed estrogen-sensitized guinea pig may be evoked by progesterone in small dosage. Hertz, Meyer, and Spielman (36) have attempted to quantitate this response for assay pur- poses and obtain measurable results with as little as 0.05 mg. per pig.
The variability has not been fully defined.
The endometrial response of the rabbit is obtained also with desoxy- corticosterone and methyltestosterone (39). It is not obtained with pregnanediol although pregnanediol may augment the activity of proges- terone (61). Since these steroids and the estrogens may be easily excluded from progesterone preparations by ordinary extraction methods progesterone bioassay presents no special problem.
When the detection of progesterone in biological specimens is sought all except the most sensitive methods are excluded due to the extremely low concentration of this hormone in most fluids and tissues. Only corpora lutea, ovaries, and placentas have yielded easily quantifiable amounts. Its excretion into urine does not normally occur, and it is reported that only 1/30,000th of administered hormone appears in the urine (38).
R E F E R E N C E S
1. Allen, E., and Doisy, E. A. Am. Med. Assoc. 81, 819 (1923).
2. Allen, E., Doisy, Ε. Α., Francis, B. F., Robertson, L. L., Colgate, C. E., Johnston, C. G., Kountz, W . E., and Gibson, Η . V. Am. J. Anat. 34, 133 (1924).
3. Astwood, Ε. B. Endocrinology 23, 25 (1938).
4. Astwood, Ε. B. Endocrinology 1 , 49 (1939).
5. Astwood, Ε. B., and Jones, G. E. S. Biol. Chem. 137, 377 (1944).
6. Bachman, C. ibid. 131, 455 (1939).
7. Bachman, C. ibid. 131, 463 (1939).
8. Bachman, C. Am. J. Obstet. Gynecol. 42, 599 (1941).
9. Bachman, C , and Pettit, D . S. J. Biol. Chem. 138, 689 (1941).
10. Barnett, J., Henly, Α. Α., and Morris, J. O. R . Biochem. J. 40, 445 (1946).
11. Butenandt, A. Z. physiol. Chem. 191, 127 (1930).
12. Butenandt, Α., and Ziegner, E. ibid. 188, 1 (1930).
13. Cartland, G. F., Meyer, R . K., Miller, L. C , and Rutz, M . H . J. Biol. Chem.
109, 213 (1935).
14. Chevallier, Α., Cornil, L., and Verdohn, J. Bull. acad. med. 114, 171 (1935).
15. Clanberg, C. Zentr. Gynakol. 54, 2757 (1930).
16. Cohen, S. L., and Marrian, G. F. Biochem. J. 28, 1603 (1934).
348 GREGORY PINCUS
17. Corner, G. W., and Allen, W . M . Am. J. Physiol. 88, 326 (1929).
18. Coward, Κ. H., and Burns, J. H. J. Physiol. 63, 270 (1927).
19. Curtis, J. M . , and Doisy, E. A. J. Biol. Chem. 91, 647 (1931).
20. Curtis, J. M . , Miller, L. C , and Witt, E. ibid. 2 1 , 119 (1937).
21. Curtis, J. M . , Witt, E., and Knudsen, L. E. Endocrinology 34, 149 (1944).
22. D ' A m o u r , F. Ε., and Gustavson, R . G. Pharmacol. 57, 472 (1936).
23. David, K. Acta Brevia Néerland. Physiol. Pharmacol. Microbiol. 4, 64 (1934).
24. de Jongh, S. E., Laqueur, E., and de Fremery, P. Biochem. Z. 250, 448 (1932).
25. Doisy, E. A. Endocrinology 30, 933 (1942).
26. Dorfman, R. I. Proc. Soc. Exptl. Biol. Med. 45, 594 (1940).
27. Dorfman, R. I., Gallagher, T. F., and K o c h , F. C. Endocrinology 19, 33 (1935).
28. Emmens, C. W . J. Physiol. 94, P2 (1938).
29. Emmens, C. W . Med. Research Council (Brit.) Special Rept. Series 234 (1939).
30. Emmens, C. W . J. Endocrinol. 2, 444 (1941).
31. Evans, H. M., and Bishop, K. S. Science 56, 650 (1922).
32. Friedgood, H. B., and Garst, J. Recent Progress in Hormone Research. Vol. II, p . 31, Academic Press, New York, 1948.
33. Furchgott, R . F., Rosenkrantz, H., and Shorr, E. Biol. Chem. 163, 375 (1946).
34. Hartman, C. G., Littrell, J. L., and T o m , J. Endocrinology 39, 120 (1946).
35. Hertz, R. ibid. 37, 1 (1945).
36. Hertz, R., Meyer, R. K., and Spielman, M . A. ibid. 21, 533 (1937).
37. Hoffmann, F., and von Lâm, L. Zentr. Gynäkol. 66, 292 (1942).
38. Hoffmann, F., and von Lâm, L. ibid. 67, 1082 (1943).
39. Hohlweg, W . ibid. 63, 1143 (1939).
40. Haskins, A. L., Jr. Endocrinology 27, 983 (1940).
41. Jayle, M . F., Crepy, O., and Judas, O. Bull. soc. chim. biol. 25, 301 (1943).
42. Jayle, M . F., Crepy, O., and Wolf, P. ibid. 25, 308 (1943).
43. Jones, R . Ν. Recent Progress in Hormone Research. V o l . II, Academic Press, p. 3, New York, 1947.
44. Kahut, L. C , and Doisy, E. A. Endocrinology 12, 760 (1928).
45. Kober, S. Biochem. Z. 239, 209 (1931).
46. Lauson, H. D . , Heller, C. G., Golden, J. B., and Sevringhaus, E. L. Endocrinol- ogy 24, 35 (1939).
47. Lloyd, C. W., Rogers, W . F., Jr., and Williams, R . H . ibid. 39, 256 (1946).
48. Lyons, W . R., and Templeton, H. J. Proc. Soc. Exptl. Biol Med. 33, 587 (1936).
49. McGinty, D . Α., Anderson, L. P., and McCullough, Ν . B. Endocrinology 24, 829 (1939).
50. McPhail, M . K. </. Physiol. 83, 145 (1934).
51. Marrian, G. F., and Gough, N. Biochem. J. 40, 376 (1946).
52. Marrian, G. F., and Parkes, A. S. J. Physiol 67, 27 (1929).
53. Mather, A. Biol. Chem. 144, 617 (1942).
54. Palmer, A. Univ. Calif. Pub. Pharmacol 1 , 375 (1941).
55. Pedersen-Bjergaard, K. Comparative Studies Concerning the Strengths of Estrogenic Substances. Oxford Univ. Press, London, 1939.
56. Pincus, G. J. Clin. Endocrinol. 5, 291 (1945).
57. Pincus, G., and Pearlman, W . H. Cancer Research 1 , 970 (1941).
58. Pincus, G., and Pearlman, W . H. Endocrinology 31, 507 (1942).
59. Pincus, G., and Pearlman, W . H. Vitamins and Hormones, 1 , 294 (1943).
60. Pincus, G., and Werthessen, Ν. T. Am. J. Physiol 120, 100 (1937).
61. Pincus, G., and Werthessen, Ν. T. ibid. 124, 484 (1938).
IX. ASSAY OF OVARIAN HORMONES 349
62. Pincus, G., and Werthessen, Ν . T . (Unpublished data).
63. Pincus, G., Wheeler, G., Y o u n g , G., and Zahl, P. A. J. Biol. Chem. 116, 253 (1936).
64. Pincus, G., and Zahl, P. A. J. Gen. Physiol. 20, 879 (1937).
65. Pratt, J. P., and Smeltzer, M . Endocrinology 13, 320 (1929).
66. Pugsley, L. I. ibid. 39, 161 (1946).
67. Pugsley, L. L, and Morrell, C. A. ibid. 33, 48 (1943).
68. Reifenstein, Ε. C., Jr., and Dempsey, E. F. Clin. Endocrinol 4, 326 (1944).
69. Report of the conference on the standardization of sex hormones. Quart. Bull.
Health Organ. League of Nations. 4, 121, 1935.
70. Report of the second conference on the standardization of sex hormones. Quart.
Bull. Health Organ. League of Nations. 4, 618, 1935.
71. Reynolds, S. R . M . , and Ginsburg, Ν . Endocrinology 31, 147 (1942).
72. Schiller, J. ibid. 36, 7 (1945).
73. Schiller, J., and Pincus, G. Science 98, 410 (1943).
74. Schmulovitz, M . J., and Wylie, Η. B. Lab. Clin. Med. 21, 210 (1935).
75. Smith, O. W . , Smith, G. V. S., and Schiller, S. Endocrinology 25, 509 (1939).
76. Sola, S. L. Rev. sud-americana endocrinol. inmunol. quimioterap. 18, 325 (1935).
77. Stimmel, B. F. J. Biol. Chem. 162, 99 (1946).
78. Stimmel, B. F. ibid. 165, 73 (1946).
79. Szego, C. M . Lancet 62, 423 (1942).
80. Szego, C. M., and Roberts, S. Proc. Soc. Exptl. Biol. Med. 61, 161 (1946).
81. Szego, C. M . , and Samuels, L. T . J. Biol. Chem. 151, 587 (1943).
82. Talbot, Ν . B., Berman, R. Α., MacLachlan, Ε. Α., and Wolfe, J. K . J. Clin.
Endocrinol. 1 , 668 (1942).
83. Talbot, Ν . B., Wolfe, J. K., MacLachlan, Ε. Α., Karusch, F., and Butler, A. M . Biol. Chem. 134, 319 (1940).
84. Thayer, S. Α., Doisy, Ε. Α., Jr., and Doisy, E. A. Yale J. Biol. Med. 17, 19 (1944).
85. Van D y k e , Η . B., and Chen, J. S. Endocrinology 25, 337 (1939).
86. Veitch, F. P., and Milone, H. S. Biol. Chem. 158, 61 (1945).
87. Venning, Ε. H. ibid. 119, 437 (1937).
88. Venning, Ε. H. Endocrinology 39, 203 (1946).
89. Venning, Ε. H., Evelyn, Κ. Α., Harkness, Ε. V., and Browne, J. S. L. J. Biol.
Chem. 120, 225 (1937).
90. Werthessen, N . T., and Baker, C. F. Endocrinology 36, 351 (1945).
91. Werthessen, N . T., and D y m e , H . C. (Personal communication).
92. Wolfe, J. K., Hershberg, Ε. B., and Fieser, L. F. J. Biol. Chem. 136, 653 (1940).
93. Zimmerman, W . Z. physiol. Chem. 233, 257 (1935).