ContentslistsavailableatScienceDirect
Journal of Molecular Structure
journalhomepage:www.elsevier.com/locate/molstr
The evaluation of the anticancer activity of the Biginelli hybrids and pharmacokinetic profiling based on their retention parameters
Jovana Ristovski (Trifunovi ´c)
a, Renáta Minorics
b, Sándor Bartha
b, Nenad Jankovi ´c
c,∗, István Zupkó
baUniversity of Novi Sad, Faculty of Medicine, Hajduk Veljkova 3, 210 0 0 Novi Sad, Serbia
bUniversity of Szeged, Faculty of Pharmacy, Department of Pharmacodynamics and Biopharmacy, Eötvös u. 6., H-6720 Szeged, Hungary
cUniversity of Kragujevac, Institute for Information Technologies Kragujevac, Department of Science, Jovana Cviji ´ca bb, Kragujevac, 340 0 0, Serbia
a rt i c l e i nf o
Article history:
Received 17 December 2021 Accepted 6 January 2022 Available online 8 January 2022 Keywords:
Biginelli chemistry Tetrahydropyrimidines Selectivity index QSRR model
a b s t r a c t
Thepresentinvestigationgivesaninsightintotheevaluationandanalysisoftheanticanceractivityofthe libraryoftheBiginellihybridsusingtheappropriateQSRRapproach.UsingtheRPTLCmethodretention parametersoftestedcompoundswereobtainedandexaminedtomeasureoflipophilicityofinvestigated molecules.The compoundswereexaminedin sevendifferent cancer celllines and theirIC50 and% of inhibitionofcellproliferationat100μMwereestablished.Theseexperimentalvaluesaswellasappro- priatemoleculardescriptorsareincludedinQSRRanalysis.Forthispurpose,thevariableselectionwas made,PCAand HCAwerecarriedout,nineMRmodelsweredevelopedand ranked.Thequality ofthe establishedmodelswas confirmedthroughinternaland externalstatistical validation.Thegoalwasto definethemaindifferencesandsimilaritiesbetweenthreegroupsofthetestedBiginellihybridsto-find outwhichmolecularfeaturesaffectlipophilicitythemostandwhicharecrucialforthedevelopmentof high-qualityQSRRmodels.Tetrahydropyrimidineswithbutyl(11)and benzylfragment(19)possessthe bestanticanceractivityandselectivity.Nowadaysmoderndesignofanactivepharmaceuticalingredient includesspecificrequirementsofrationalizationtoadaptphysicochemicalcharacteristics,pharmacologi- calactivity,andsafetythroughstructuralchangesofthemolecule.Webelievethatthedevelopedprofile isastepforwardinBiginellichemistryandcouldbeusefulinthefuturesynthesisofnovelBiginelli-based compoundswithsignificantlyimprovedactivities.
© 2022 Elsevier B.V. All rights reserved.
1. Introduction
Pyrimidines act as a potential framework for DNA and RNA, elucidating itsimportanceindrugdiscoveryanddevelopment[1]. From thepyrimidines family very importantare Biginelli hybrids tetrahydropyrimidines-THPMs (former name-dihydropyrimidines).
TheywereoriginallysynthesizedintheXIXcenturyinamulticom- ponent chemical reactionproposed by Pietro Biginelli [2,3].Since then,duringtheoptimizationofthischemicalreaction,conditions were modifiedseveral timeswhich resulted in the generation of higheryields,improvedenantioselectivities,andinclusionofgreen methodologies [4]. Thesescaffolds have attractedimposing inter- estofmedicinalchemistsconsideringtheirdiversetherapeuticand pharmacological properties. Straight forward synthesis of THPMs led to the discovery ofmany significant products such asantidi- abetic, calcium channel blockers,[5] adrenergic receptor antago-
∗Corresponding author.
E-mail address: nenad.jankovic@kg.ac.rs (N. Jankovi ´c).
nists,anti-inflammatory, antiviral,antioxidant, andanti-SARS TH- PMsagents[6].In1999Mayeretal.publishedtheirwork regard- ingmitotickinesininhibitorcalled„Monastrol” andsincethenthe interestfortheanticanceractivityofTHPMsdoesnotsubside[7]. From the beginning ofthe Biginelli era, therewere thousands of Biginelli-like compounds published out to date. Eventhough the Biginelli reaction was produced a huge number of molecular li- braries with broad spectra of activities, among them nonewere pharmacokineticprofiled.
Lipophilicityrepresentsa physicochemicalparameter -thatde- termines the ability of chemical compounds to dissolve in fats, oils, lipids, polar or non-polar solvents, and different body flu- ids. Specifically, lipophilicity is firmly linked to the behavior of moleculesinthebiologicalmediumthereby directlyregulatingits capability forabsorption, distribution, metabolism,excretion, and toxicity (ADMET). It has also been found to affect several com- plexpharmacokineticparameterssuchaspermeabilityinthegas- trointestinaltract, passagethroughthe blood–brainbarrier,pene- tration through different tissue membranes, interaction withen-
https://doi.org/10.1016/j.molstruc.2022.132373 0022-2860/© 2022 Elsevier B.V. All rights reserved.
zymes and efflux pumps, andbinding to plasma proteins. Parti- tioncoefficient(P)ordistributioncoefficient(D)representsthera- tioofconcentrationsofachemicalcompoundinanoctanol/water solventsystematequilibrium.Althoughoctanol/waterpartitioning establisheditselfastherelevantsystemothersolventsystemsmix- tureshavebeenwidelyexaminedaspotentialalternatives.Consid- eringthe importance oflipophilicity, thepartition coefficient can be measured experimentally in severaldifferent ways: by shake- flaskmethod,employingchromatographictechniques,orusingpH titration [8]. Due to its key importance, some computational ap- proaches havebeen developedto calculateappropriate logP val- ues.Variousprogramsrelybasedonthesumofthecontributions (
π
)ofthemolecularfragments,Abraham’slinearfree-energyrela- tionship,quantumchemicalmethodsorimplycombinedapproach [8].Experimentallyobtainedorcalculated logP valuesoftenpro- videgoodcorrelationswithADMEpropertiesatleastwithinacon- generic seriesof compounds.Nevertheless,log P alonecannot be competent in the prediction of ADME properties for structurally differentcompounds.Reversed-phase thin layer chromatography (RP TLC) repre- sentsa widely appliedchromatographic methodthat canprovide lipophilicity estimation of many compounds with quantitatively comparable, accurate, andreproducible retention data [9,10]. Al- though high-performanceliquidchromatography(HPLC)possesses many advantagessuch asgood resolution,speedof analysis, sen- sitivity, andreproducibility itcanbe moreexpensiveandcompli- cated in certain cases and can lead to more environmental pol- lution due to the utilizationof huge amount/volume of different solventsandchemicals. Havinginmindallaforementionedinthis researchRPTLCmethodwasappliedtoestimateretentionparam- etersofBiginellihybrids.
Estimatedlipophilicityparametersarefurtherexploitedinsev- eral analysisof molecular structureproperties relying onchemo- metricmethodologies.Thence,thequantitativestructureretention relationship (QSRR) method hasbeen considered adequate to es- tablish a specific strategy and methodology of molecular prop- ertypredictions.Thisapproachisconsideredconvenient,especially from an overall chemometric perspective because it ensures the besttestingoftheapplicabilityofspecificstructuralparametersfor propertydescription.QSRRapproachcanbealsofurtheremployed intheidentificationofthemostsuitablemolecular descriptors,in the prediction of retention parameters of new analytes, in bet- ter understanding ofmolecularseparation undergivenchromato- graphicconditions,andforevaluationofdifferentphysicochemical propertiesofexaminedmolecules.[11]
Theobjectiveofthisworkistoinvestigatethelipophilicitypa- rameters obtained in the RP-TLC chromatography and their pos- sible application in the better rationalization of pharmacological propertiesofBiginellihybrids,and,therefore,theirapplicabilityin futureQSRRstudiesrelatedtothistypeofscaffolds.Duringthere- search, acomprehensiveanalysisofselectedcompoundswasper- formed withtheaimto providea deeperinsightinto themolec- ular properties and their biological activities. To reveal similari- ties/dissimilaritiesbetweeninvestigatedTHPMsmultivariatestatis- ticalanalysiswasperformed. Throughthisapproach mainmolec- ulardescriptorsthataffectlipophilicityweredetermined.Also,the relationshipbetweenchemicalstructureandanticanceractivityof Biginellihybridsisrevealed.Forthepredictionofanticanceractiv- ityofselectedBiginellihybridsappropriatemultiplelinearregres- sionmodels(MR) weredeveloped.Tocompareexperimental with thecomputationalapproachinestimationofanticanceractivityof selected compoundsarelatively novelmethodnamedasthesum ofrankingdifferences(SRD)wasemployed.[12]Thismethodology has beensuccessfullyappliedforthe comparisonofobtainedMR modelswithexperimentallyestimateddataandtocompareclassi- calwithnovelchemometricmethodologies.
ConsideringthesignificanceofTHPMsandourpreviousactivity inBiginellichemistry,[13–18]twenty-fourcompoundswereinves- tigated(Table1)intermsoftheiranticanceractivityandpharma- cokinetics.
2. Resultsanddiscussion
2.1. ChromatographicanalysisofselectedBiginellihybridsandits relationshipwithmolecularlipophilicity
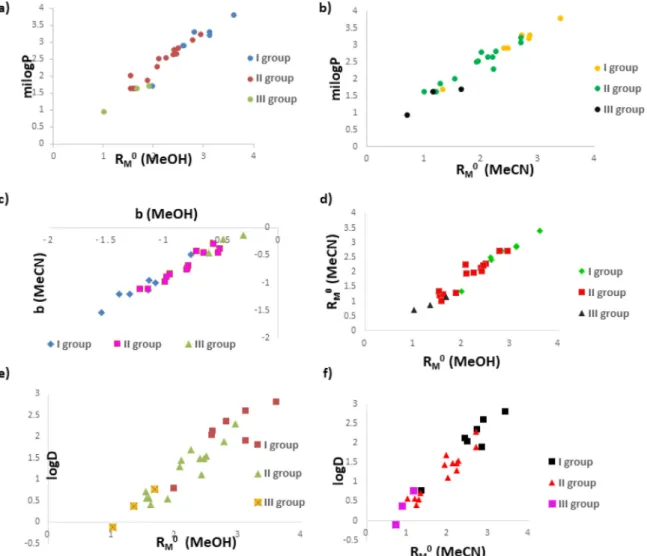
Considering the limitations of normal phase chromatography systems and analyzing the lipophilic nature and chemical struc- tureofselected Biginellihybrids ouranalyteswere examinedus- ing RP-C18 thin-layer plates. Two types of mobile phases were applied, one phase wasbased on MeOH and the second one on MeCN, each with the same ratio of organic solvent and water ((MeCN/MeOH)=0.5–0.7v/v).Incomparisonwithnormal-phase systems,muchbetterretentionbehaviorwasnotedusingreversed- phasesystems.Itwasprobablyduetochemicalnatureofselected compoundsandtheirinteractionswithpolarstationaryphase.The groupofBiginellihybridsstudiedinthisworkincludedmolecules with similar structures, sizes, and polarities, so it was expected that they have similar chromatographic behavior. Thus, the frac- tion ofMeOH andMeCN, forwhich a linearrange wasobtained forretentionparameters, rangedbetween50 and70%andan in- crementof5%wasappliedtoachievethefivespecifiedconcentra- tions.TheRMvaluesdecreasedlinearly,withanincreasingconcen- tration ofMeOH/MeCNin themobilephase. Thesevaluesextrap- olatedto0%ofMeOH/MeCNgavetherelativelipophilicityparam- eter(RM0)values,whichcharacterizethepartitioningbetweenthe non-polarstationaryandpolarmobilephases. After extrapolation ofRM values,obtainedRM0 valuesrepresentretentionoftheana- lyzedcompoundsinpurewater.TheRM0valuesreflectlipophilicity ofexaminedmoleculesandtheslope(b)isrelatedtohydrophilic surfacearea ofthesecompounds. The established linearrelation- ship between the relative lipophilicity parameter (RM0) and the slope (b) (Fig 1.) ensured us to spot congeneric compound sub- classes in the set of studied Biginelli hybrids. Examined THPMs moleculesoftwenty-fourhybrids(Table1)areseparatedintothree groupsaccordingto their fragmentsat C5position: ethyl ester (I group),methylester(II group)andacetyl(IIIgroup).Compounds possesssimilarlogPvaluesbutdifferincalculatedADMEandex- perimentallyobtainedanticanceractivities.Thedifferenceinmolar mass(220.23 – 455.35) andsolventaccessible surfacearea ofall hydrophobic atoms (58.64 – 112.20) indicate the presence of di- versesubstituentswhichcouldbethereasonforvariousbiological effectsofexaminedmolecules.
Thestrong lineardependenceofretentionparameters through the MeOH/MeCN fraction withthe calculated milogP values was demonstratedbythevaluesofR2of0.95and0.96(TableS1,Fig.1a and1b). Also, correlation betweenretention constants(RM0) and slope(b)isstatisticallysignificantwithR2valuesof0.9518(Fig.1c) and0.9533(Fig.1d)respectively.Furthermore,consideringthecor- relationbetweenRM0 parametersestimatedbyusingboth mobile phasesandlogDpH=7.4(R2MeOH/H2Ois0.9185(Fig.1e)and R2MeCN/H2Ois0.9013(Fig.1f)aswellasinthecasewithmiLogP can be indicated that RM0 values of MeOH/H2O and MeCN/H2O couldbeappliedaschromatographiclipophilicityparametersofin- vestigatedBiginellihybrids.
Inthisstudy,twochromatographicsystemswereexamined.The firstisbasedonmethanol,thesecondisbasedonacetonitrilewith the sameratio ofwater. Using MeOHaspolar protic solventob- tainedlipophilicityparametersofRM0valuesarehigherthaninthe casewhenMeCNaspolaraproticsolventwasapplied.Thehighest numberofspotsandseparationefficiencywasobservedby using acetonitrile–water with 60% of MeCN. From the qualitative point
Table 1
The chemical structures of the studied THPMs ( 1 –24 ).
.
Compound R1 R2 R3 R4 R5 Group
1 COOCH 2CH 3 H H H S I
2 COOCH 2CH 3 OCH 2CH = CHCH 3 OCH 3 H O I 3 COOCH 2CH 3 OCH 2C(CH 3) = CH 2 OCH 3 H O I 4 COOCH 2CH 3 O(CH 2) 4Br OCH 3 H O I 5 COOCH 2CH 3 O(CH 2) 5Br OCH 3 H O I
6 COCH 3 OH OCH 3 H O III
7 COOCH 2CH 3 OCH(CH 3) 2 OCH 3 H S I
8 COOCH 3 O(CH 2) 2CH 3 OCH 3 H O II
9 COOCH 3 OCH 2CH = CHCH 3 OCH 3 H O II 10 COOCH 3 OCH 2C(CH 3) = CH 2 OCH 3 H O II 11 COOCH 3 O(CH 2) 3CH 3 OCH 3 H O II
12 COOCH 3 O(CH 2) 3Br OCH 3 H O II
13 COOCH 3 OCH 2CH = CH 2 OCH 3 H O II
14 COOCH 3 OCH 3 OCH 3 H O II
15 COOCH 3 OCH 2CH 3 OCH 3 H O II
16 COOCH 3 OCH 2COOCH 2CH 3 OCH 3 H O II
17 COCH 3 – – – O III
18 COOCH 3 H H H O II
19 COOCH 3 OCH 2Ph OCH 3 H O II
20 COCH 3 NO 2 H H O III
21 COOCH 2CH 3 OH OCH 3 H O I
22 COOCH 2CH 3 OCH 3 OCH 3 H O I
23 COOCH 3 OCH 3 OCH 3 OCH 3 O II
24 COOCH 3 Cl H H O II
of view, simple RP-C18 platesdeveloped in a mobile phase con- taining MeCN/H2O((MeCN) = 0.6) are sufficient forseparation ofalltestedBiginellihybrids.Thismethodcouldbeparticularlyin- terestingasanidentificationstepinthequalitycontrolduringthe synthesis ofdifferentBiginellihybrids.Modificationsofthechem- ical structure ofBiginelli hybrids by introducing different groups that influencelipophilicity canlead to change ofADMET proper- tiesthatarealsoaffectedbylipophilicity.Becauselipophilicitycan be experimentally measured aswell ascalculated it allows com- biningtheoreticalandexperimentalapproachesincomparisonand testingof thesemethodsandimprovementoffuturesteps inthe designofnovelBiginellihybrids.
2.2. Multivariatestatisticalanalysis
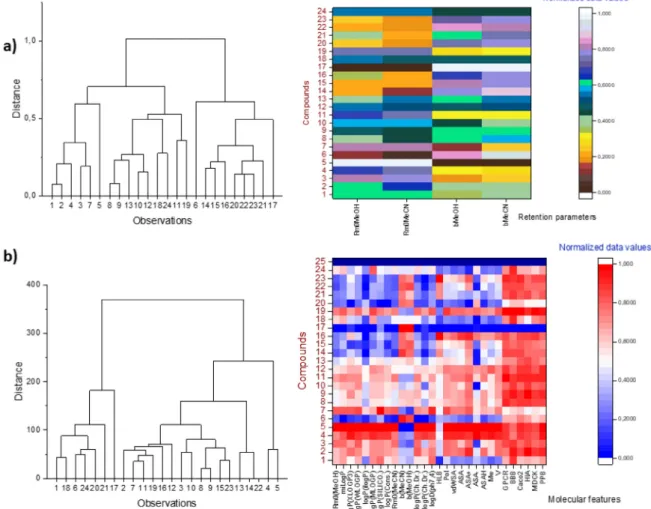
InHCAanalysisareincludedexperimentallyobtainedretention parametersaswellasmoleculardescriptorsrelatedtoADMEchar- acteristics ofstudied moleculestoreveal similaritiesanddissimi- laritiesbetweenthreegroupsofTHPMs.Inadditiontotheimple- mentation ofdendrograms, results are also presented using heat maps whereare the lowestvalues depictedindark red/blue and thehighestingray/redcolor(Fig.2).
Clustering of compounds regarding retention parameters indi- cates the good separation betweenmolecules with a very small
numberofoverlapsbetweenthem (Fig.2a).Group Iiswellsepa- ratedwithinthefirstclusterwithcompound21thatrepresentsex- ceptionandbelongstothesecondcluster.Thereasonforthiscould beinsimilarityofitsretentionparameterstothesecondgroupof molecules.Thiscompoundaccordingtoits chemicalstructure be- longs togroupIbutconsidering chromatographicbehaviorit isa memberofgroup II.Asapartofsecond cluster,itcanbe spotted thirdgroupofexaminedBiginellihybridswhichpossessthelowest valuesof retentionparameters. Compound 17ofgroup III can be markedasoutlier,probablyduetopresenceoffurylgroup which makes itspecificamongthe studiedBiginelli hybrids.Thesecond group ofstudiedmolecules isdeployedwithin bothclusterscon- sideringits chromatographicparameters whichare in themiddle ofthescaleofpresenteddatavalues.
After performing HCAanalysison thedata setwhich includes retentionparameters, physicochemicaldescriptors, andADMEde- scriptors,clusteringresults(Fig.2b)haveshowncertaindifferences fromtheresultsobtainedinHCAwhenonlyretentionparameters were included (Fig 2a). From group I ofcompounds molecules 1 and21are placedindifferentclusterfromtheother membersof this group. The reason could be due to specific pharmacokinetic properties which are more like the compounds fromthe second cluster. Compounds that belong to group III are arranged in the second cluster which includes 17 molecules that possess similar
Fig. 1. Retention parameters (R M0) of studied Biginelli hybrids obtained in two solvent systems MeOH/H 2O (a) and MeCN/H 2O (b) in correlation with milogP. Slope b of two solvent systems in mutual correlation (c) and retention parameters in mutual correlation (d) as well. LogD in correlation with retention parameters R M0
(MeOH)(e) and R M0 (MeCN)(f).
ADME characteristics. Moleculeswith the best antitumoractivity belong to this cluster and show significant similarity in physic- ochemical andpharmacokineticaspects. Appropriate pharmacoki- netic profiles ofinvestigated molecules are ofcrucial importance forfutureevaluationoftheirpossibletherapeuticapplication.Con- sideringthisfactprofoundHCAanalysisenablesinsightintosim- ilarities/dissimilarities between compounds regarding their phar- macologicalfeatures (Fig.2b).Molecules11,12,and19whichare labeledasthemostpotentcandidatespossessalsosatisfyingphar- macokinetic propertiesandwillbesubjectedtofurtherstudiesto examinetheirtherapeuticpotential.
InordertoreducethedimensionalityofourdatasetsPrincipal component analysis was performed and two components model wasacquiredwith94%ofthetotalvariance.ExtractedEigenvectors and Eigenvalues of the correlation matrix are presented in Elec- tronicSupplementaryInformation(ESI)(TableS6andS7).Thebi- plot represents both the loadings andthe score fortwo selected components in parallel. Investigated Biginelli hybrids were ana- lyzed in thecontext ofthe lipophilicityandchromatographicbe- havior(Fig3a)calculatedphysicochemicaldescriptors(Fig.3b)and pharmacologicalproperties(Fig3c).ThebiplotpresentedinFig.3a suggeststhat alongthe PrincipalComponent1axis,whichrepre- sents 91.11% of total variability, group I shows higher lipophilic- ityvaluesthan groupIII whichislocated ontheopposite side of PrincipalComponent1axis.AlongsidethePrincipalComponent2
axis,whichcovers 2.98%oftotalvariability,smallgroupingofthe compoundsoftheIIseriescanbespotted.Itcanbeobservedthat retentionparameters(RM0 MeOHandbMeCN)stronglyinfluence PrincipalComponent2asitispresentedinthebiplot(Fig.3a).Re- tentionparametersalsohavesignificantimpactonPrincipalCom- ponent1,however,itisevidentthattheseparationoftheseriesII alongPrincipalComponent2ispredominantlybasedon b(MeCN) values.
ThesecondPCAmodelisbasedonthephysicochemicalmolec- ularpropertiesofinvestigatedcompounds.Thisestablishedmodel covers92.13%ofthetotalvariability.ThebiplotpresentedinFig.3b suggeststheadequatedistributionoftheBiginellihybridsbetween thePCA1andPCA2axis.ThedetailsregardingsecondPCAmodel arepresented inTablesS7 andS8.Similarly, totheprevious PCA model Group I of examined molecules is placed on the positive side and group III on the negative side of PCA 1 axis. All in- cludedphysicochemicalparametersexpressstronginfluenceonthe PCA1,butontheother side,ASAminus andHLBstronglyaffected PCA2andshow lessinfluence onPCA1. Compound 1 showssim- ilar physicochemical characteristics with group III of compounds whichcan be a consequenceof thepresented sulfur atom inside thismolecule. Compound 24fromII group alsopossesses similar physicochemical effects with group III which can also be due to thepresenceofchlorineatominthemolecule.
Fig. 2. HCA analysis presented in dendograms and heating maps. Clustering of molecules according to their retention behavior (a) considering parameters R M0and b.
Clustering of molecules according to their retention parameters, calculated log P values, physicochemical descriptors and ADME descriptors (b).
In order to investigate relations between experimentally ob- tained (%INH) values of analyzed compounds in seven different cell lines, retention parameters, and calculated pharmacokinetic descriptors thethirdPCAanalysiswasperformed(Fig.3c).Details regarding the thirdPCA modelcan, also,be found in ESI(Tables S10andS11).ThisPCAmodelexplains79.64%ofthetotalvariance, withthe twosignificant PCA.Analyzingbiplotasa resultofPCA, similarities, ordissimilaritiesamongthedatacanbe observed.All experimentally obtaineddata (%INH values andretention param- eters) possesspositiveinfluenceonPC1.Pharmacokineticdescrip- tors HIA,PPB, andCaco2showalsopositiveinfluenceonPC1and they are in good correlation with experimentally obtained data.
The results ofthePCA analysissuggest whichmolecular descrip- tors are suitable to be included infurther chemometrics analysis and which should not be considered during the development of appropriateQSRRmodels.
The results ofthe multivariate analysisindicate which molec- ular properties of studied Biginelli pyrimidines should be partic- ularlyincluded infurtherchemometric analysis.Furthermore,the datafromHCAandPCAanalysisemphasizespecificmolecularde- scriptors whichare responsible forthe main differencesbetween examinedcompounds.Structuralchangesofthemoleculescanaf- fect specific molecular properties e.g. inductive andsteric effects and havealso an impacton the chromatographicbehavior of in- vestigated molecules.Aliphatic andphenylgroupsarevoluminous andbecauseofthestericeffect,theyhinderaccessofdonoratoms to silanol groups on the stationaryphase. According to HCA and PCA analysis, it could be noted that the groupingofinvestigated
moleculesintothreeseriesisparticularlybasedonthetypeofsub- stituentsattachedtoTHPMmoiety.ObservedclusteringofBiginelli hybridsalong PC1axisinthe contextofRP TLCshowsexpressed grouping of molecules in relation to the presented substituents withtheaim tofavor hydrophobic interactions inthischromato- graphicsystem.
2.3. QSRRmodelingofanticancerandretentionbehaviorofBiginelli hybridsbyMRapproach
In establishing QSRR model Multiple linear regression (MR) analysis was applied. Performed MR is based on a variety of calculated molecular descriptors selected by the stepwise vari- able subset selection procedure. The stepwise selection was uti- lizedtodeveloparegressionequationfortwenty-fourcompounds and molecular descriptors are chosen based on their impact on theretention parametersofinvestigated moleculesunderthe ap- plied chromatographic conditions. The regression equations in- cluded three parameters:distribution coefficient (log D, pH 7.4), thesolventaccessiblesurfaceareaofallhydrophobicatoms(ASA- H),andmolecularweight(Mw)oftheexaminedcompounds.Mod- eling ofretentionparameters ofthesemolecules asafunction of the theoretically derived descriptors was established by MR. MR modelsarerepresentedbyequations:
MR1:R0M(MeOH)=0.9677
(
±0.2893)
+0.7363(
±0.0768)
LogD−0.0014(
±0.0011)
ASA-H+0.0029(
±0.0019)
MwFig. 3. Results of PCA analysis for first two components considering a) retention parameters and calculated logP values b) physicochemical descriptors, c) retention parameters,%INH and pharmacokinetic descriptors. .
Table 2
Results of statistical analysis for MR1 and MR2 models.
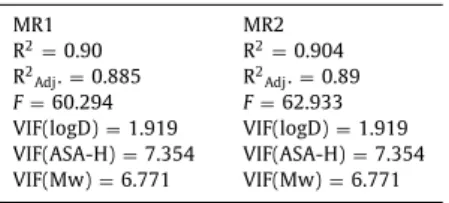
MR1 MR2
R 2= 0.90 R 2= 0.904 R 2Adj. = 0.885 R 2Adj. = 0.89 F = 60.294 F = 62.933 VIF(logD) = 1.919 VIF(logD) = 1.919 VIF(ASA-H) = 7.354 VIF(ASA-H) = 7.354 VIF(Mw) = 6.771 VIF(Mw) = 6.771
Table 3
Cross validation summary for MR1 and MR2 models.
CV −0.008 0.08
PRESS −0.2 0.2
SS T 9.21 12.52
R 2(pred.) 1.02 0.98
Table 4
Variable importance projection-VIP values of MR1 and MR2 models.
MR1 MR2
logD 1.2 1.2
ASA-H 0.9 0.9
Mw 0.9 0.8
MR2:R0M(MeCN)=0.634
(
±0.33)
+0.864(
±0.087)
LogD−0.0009
(
±0.0013)
ASA−H+0.00178(
±0.0022)
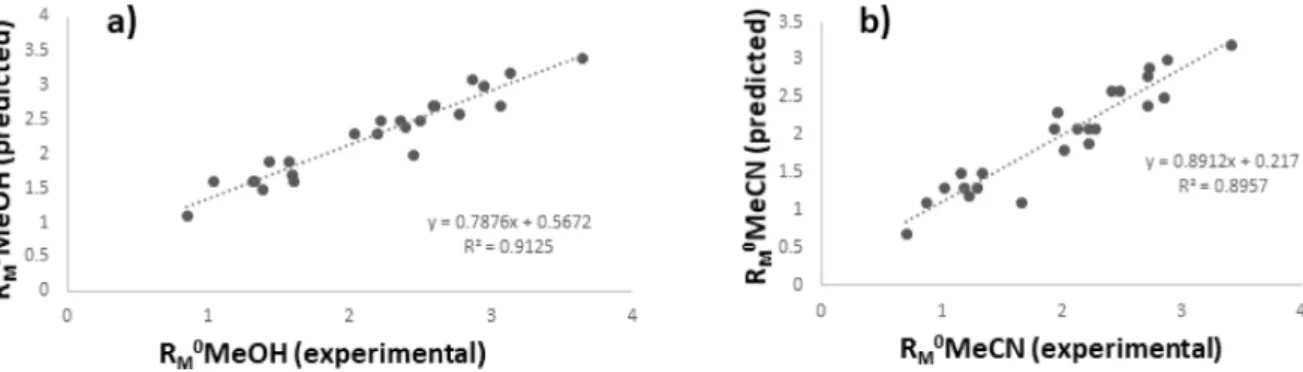
MwInternalandexternalvalidationwasperformedtodevelopsta- tisticallyvalidmodels forthe retentionparameters prediction.To conductexternalvalidationdatasetwasdividedintotwosets,cal- ibration set that includes nineteen samples andan external test set withfive samples.Obtained results indicate strong a correla- tionbetweenretentionparametersandselectedmoleculardescrip- tors.Predictedretentionvaluesareingoodagreementwithexper- imentallyobtained(Fig.4).Thisisconfirmedbydeterminationco- efficientsoftheestablishedmodes, interceptvaluesaround0and slope valuesaround 1.Thestatistical parameters oftheMR1 and MR2modelsarepresentedinTable2.EstablishedMRmodelsshow significantlyhighcorrelationcoefficientsandlowvarianceinflation factorvalues (VIF). The intercorrelation ofthe selectedmolecular descriptorswasexamined andobtainedVIFparameters arelower than tenwhich exclude the multicollinearity phenomenon.F-test valuesshowgoodfittingoftheMRmodelstoadataset.
In this work leave-one-out, cross-validation method was ap- pliedtoestimatetheperformanceofestablishedMRmodels.Itin- cludes a single observationas validation data andleaves the re- mainsastrainingdata.Cross-validationerror(CV),predictedresid- ualerror sumofsquares(PRESS), the sumofsquares total(SST), andpredictive R-square(R2(pred.)) are presentedin Table3.Pre- dictiveR-square is abetter measure ofthepredictive powerof a regressionmodelthanR-square.Variableimportanceinprojection (VIP) (Table 4) value estimates the importance of each variable.
Variableisconsideredasimportantifits VIPvalueisgreaterthan 0.8.
To develop QSRR models with significant prediction ability molecular descriptors were selected as previously described. Ex- perimentallyobtainedretentionparametersareingoodcorrelation (R2≥50)withexperimentallyobtainedanticanceractivitiesofBig- inellihybrids.Additionally,thesamemoleculardescriptorsthatare includedinMR1andMR2were usedfordevelopmentofMR4–9 models.OnlyinMR3modeldesignHIAandPPB%descriptorswere
Fig. 4. The correlation between experimentally obtained retention values and predicted retention values using a) MR1 and b) MR2 model.
Table 5
Established MR3–9 models.
Multiple Linear Regression Models
MR3 IC 50(HeLa) = 20.028( ±79.498) + 16.512( ±9.18)R M0
(MeOH)−0.526( ±0.94)HIA + 0.674( ±0.372)PPB MR4 IC 50(SiHa) = −66.395( ±16.539) + 0.514( ±4.934) R M0
(MeOH)+ 0.039( ±0.07)ASAH + 0.218( ±0.121)Mw MR5 IC 50(A2780) = −110.9( ±26.251) + 22.523( ±7.832) R M0
(MeOH)+ 0.046( ±0.112)ASAH + 0.224( ±0.192)Mw MR6 IC 50(C33A) = −89.179( ±23.558) + 14.763( ±7.028) R M0
(MeOH)+ 0.024( ±0.1)ASAH + 0.25( ±0.17)Mw MR7 IC 50(MCF7) = −79.711( ±15.350) + 14.926( ±4.579) R M0
(MeOH)+ 0.0665( ±0.065) ASAH + 0.181( ±0.112)Mw MR8 IC 50(T47D) = −75.179( ±15.723) + 10.8( ±4.69)R M0
(MeOH)+ 0.001( ±0.067)ASAH + 0.279( ±0.115)Mw MR9 IC 50(MB231) = −108.524( ±18.979) + 6.283( ±5.66) R M0
(MeOH)−0.01( ±0.08)ASAH + 0.394( ±0.139)Mw
Table 6
Results of statistical analysis for MR3–9 models.
Parameters MR3 MR4 MR5 MR6 MR7 MR8 MR9
R 0.882 0.811 0.852 0.82 0.917 0.89 0.87
R 2 0.778 0.658 0.726 0.68 0.84 0.79 0.77
R 2adj. 0.745 0.61 0.685 0.64 0.817 0.76 0.74
F 23.393 12.832 17.696 14.602 35.247 25.477 22.01
p 9.5 ×10 −7 6.7 ×10 −5 7.55 ×10 −6 2.86 ×10 −5 3.55 ×10 −8 4.91 ×10 −7 1.51 ×10 −6
VIF 7.29R M0
2.18HIA 5.64PPB
1.82 R M0
7.13ASAH 6.82Mw
1.82 R M0
7.13ASAH 6.82Mw
1.82 R M0
7.13ASAH 6.82Mw Table 7
Cross validation summary for MR3–9 models.
Factors MR3 MR4 MR5 MR6 MR7 MR8 MR9
CV −0.04 0.004 −0.004 −0.008 −0.01 0 0.004
PRESS −0.1 0.1 −0.1 −0.2 −0.2 0 0.1
SS T 13,233.5 9893.95 31,143.197 7033 18,320.66 14,741.19 19,161.06
R 2(pred.) 1 0.99 0.99 0.99 0.99 1 0.99
selectedwhichisinaccordancewiththePCA3resultsthatsuggest significant influence ofthese descriptors onretention parameters (RM0(MeOH)andRM0(MeCN)).AsaresultofMRanalysis,seven(MR3- MR9)statisticallysignificantmodelsweredeveloped(Table5).
Analyzingestablished MR3–9 modelsit canbe concludedthat allphysicochemicalparameters(RM0(MeOH),ASAH,andMw)havea positiveinfluenceonthe%INHvalues.Regressioncoefficientsofthe RM0(MeOH)valuesindicatethatretentionparametershaveagreater influenceonanticanceractivitythanotherphysicochemicalparam- etersthatformthemodels.HIAdescriptorfromMR3modelhasa negativeinfluenceonthepredicteddatawhilePPBmanifestposi- tiveinfluenceontheanticancerpotentialofanalyzedcompounds.
As in the case of previous MR models, the MR(3–9) models wereevaluatedbycomparingtheexperimentalandpredicteddata (Table S12). According to R2 andR2adj. valuesall developed MR modelsindicateahighcorrelationbetweenthevariables(Table6).
Also, CV error of the anticanceractivity prediction is in allowed range(close tozero)(Table7). HighvaluesofR2adj. (in therange from0.61to0.81)(Table6)andPRESSvalueclosetozero(Table7) forall seven modelsindicatethe goodpredictiveabilityofestab- lished MRmodels. High F values (Table 6) verifythe good fit of
thedata.According toVIFvalues (Table6), multicollinearity isin theacceptable range(VIF<10) andVIPvaluesare higherthan0.8 (Table8).
2.4. MRmodelsranking
InordertorankestablishedMRmodels,SumofRankingdiffer- ences(SRD)methodwasapplied.Thiscomparingmethod isnon- parametric androbust incommonsense. SRD representsan easy tool to evaluate different QSRR models: the smaller is the sum thebetter is themodel.In theranking ofdeveloped MRmodels, amatrixbasedonexperimentallyobtainedresults(INH%valuesof twenty-fourcompounds determined in7 differentcell lines) was appliedasreferent rank.Thegoalwasto rankthedevelopedMR modelscomparing predictedvalueswithexperimentally observed IC50 values. The ranking was validated using simulated random numbersforcomparisonofranks.Theresultsarebestseenwitha figure(Fig.5);themodelsappearingontheleftsideoftheGaus- siancurve aremostsimilartothereferentrank; thus,theperfor- manceofMR5modelshouldbemostrepresentativeinthisranking procedure.BasedonresultsshowninFig.5ModelsMR6andMR7
Table 8
Variable importance projection values of MR3–9 models.
MR3 MR4 MR5 MR6 MR7 MR8 MR9
R M0(MeOH) 1.1 0.8 1 1 1 0.9 0.8
HIA 0.8
PPB 1.1
ASAH 1.1 1 1 1 1 1
Mw 1.1 1 1 1 1.1 1.1
Fig. 5. MR3-MR9 models ranked by SRD method. The statistical characteristics of Gaussian fit are the following: first icosaile (5%), XX1 = 148; first quartile, Q1 = 174; median, Med = 190; last quartile, Q3 = 208; last icosaile (95%), XX19 = 232.
have thesamerankandMR3 modelis thefarthestfromthe ref- erent rankthan all other models. The proximityof obtainedSDR valuesindicatesthesimilarityoftheestablishedMRmodels.
Thegoaloftheconductedcorrelationanalysiswastodetermine thepossibilitytopredicttheanticanceractivityofinvestigatedBig- inelli hybrids using their retention parameters. It is very impor- tant to emphasize that developed models havea very good pre- dictivecapabilityrelativetobothcalibrationandexternaltestsets whichwasconfirmedthroughcross-validation.ObtainedMRmod- elscouldbeusedinappliedconditionsinordertoavoidexpensive andtime-consumingmolecularbiologytests.
2.5. Antiproliferativeactivity
Here we evaluated the in vitro antiproliferative capacity of twenty-four Biginelli hybrids withdifferent substitutionsatposi- tions R1,R2,R3,R4,andR5onapanelofhumanadherentcancer cell lines.Molecularstructures oftestedBiginellihybrids arepre- sentedinTable1.Thecompoundsweretestedagainstbreast(MCF- 7, MDA-MB-231, and T47D), ovarian (A2780) and cervical (HeLa, SiHa,andC33-A)carcinoma celllines.Additionally,thetumor se- lectivity ofthemosteffectivecompounds wasalsodeterminedby usingnon-cancerousmouseembryofibroblast(NIH/3T3)cells.
In general, amongthetestedcancer celllines, SiHacellswere theleastsusceptibletotheexaminedBiginellihybrids:onlythree compoundspossessedIC50 valueslowerthan100μMandmostof the compoundsexerted weak (lowerthan20%)growthinhibitory effectoncellproliferation(Table9).However,ourpositivecontrol 5-FUinhibitedSiHacellproliferationata50%levelintheconcen- tration of19.2 μM.On the other hand, ourcompounds inhibited themosteffectiveproliferationofHeLacells.Morethanhalfofthe testedtetrahypyrimidines evokeda considerablystrongerantipro- liferativeeffectthan5-FUwhichwasabletoevokeaveryweakin- hibitoryeffectonHeLacellproliferation.Basedontheantiprolifer- ativeeffectsofBiginellihybridsagainstthetestedcancercelllines
asusceptibilityorderofcelllinescanbedefined:SiHa<MDA-MB- 231<A2780<C33A<T47D<MCF-7<HeLa.
AccordingtothelengthandtypeoftheO-alkylchainasR2sub- stituent,agroupofcompoundscanbedeterminedincludingcom- pounds8,11,15,16,and19.Theseanalogsdifferonlyinthechar- acteristicoftheirO-alkylfragmentasR2substituentofthephenyl ring,therefore,regardingtheir growthinhibitorydata,the impact ofthesegroupsontheantiproliferativeactivityofthetestedcom- poundscanbedetermined.Compound15canbeconsideredasthe basic compound ofthis group. This pyrimidine derivative with a methoxy function at position R2 and hydroxyl group at position R3didnotexertanygrowthinhibitoryeffectonthetestedgyneco- logicalcancercelllines.Then,compounds8and11containgroups withthreeandfourcarbonsatpositionR2,respectively,anditgen- eratedhighercellgrowthinhibitorypotential.Moreover,11isone ofthemosteffectiveBiginellihybridwithlowIC50 valuesonHeLa (6.0μM), MCF-7(8.3 μM),andT47D(7.9μM) cell lines(Table9).
The two other derivatives belonging to this group differ in the characteristic of their substituents at position R2 of phenyl ring.
Compound 16 bears OCH2COOCH2CH3 group which cannot con- siderbeingbeneficialregardingantiproliferativeactivitybecauseit exertedcellgrowthinhibitoryeffectwithmaximum30%.Molecule 19containsabulkyO-benzylgroupwhichincreaseditsantiprolif- erativeactivityonallinvestigatedcancercelllinesexceptforSiHa cellscompared to 15,the basic derivative ofthis group. The cal- culatedIC50 valueonHeLa cellsofthisBiginellihybridis7.7μM, thereforeitistheothermosteffectivecompoundamongthetested derivatives(Table9).
Therefore,twomethoxygroupscanbefoundasR2andR3sub- stituents in compounds 14 and 22, whereas compound 23 pos- sessesthreemethoxygroupsasR2,R3,andR4substituents.Based on their antiproliferative data it can be concluded that two or three methoxygroups onphenyl ring are not beneficial, because iteliminatedthecellgrowthinhibitoryactivityofthecompounds compared tothat of 19which containsone methoxyandone O-
Table 9
Antiproliferative properties of Biginelli hybrids.
Comp. Conc.( μM)
Growth inhibition;%±SEM [calculated IC 50value;μM] ¥
HeLa SiHa C33A A2780 MCF-7 T47D MDA-MB-231
1 30
100 ║
–∗
50.59 ±0.28 [99.1] #
– –
– –
– –
20.95 ±1.19 33.40 ±0.14
–
35.14 ±3.63 – –
2 30
100 47.37 ±0.86
70.52 ±1.09 [28.8]
–
26.20 ±0.76 36.08 ±1.40 55.64 ±0.99 [69.3]
30.71 ±1.99 87.82 ±1.61 [49.9]
39.74 ±1.28 68.68 ±2.81 [35.8]
26.07 ±1.33 64.22 ±1.39 [64.6]
36.61 ±3.58 44.35 ±2.34
3 30
100
51.07 ±1.75 70.49 ±0.88 [29.3]
– –
38.83 ±4.02 54.57 ±1.64 [74.5]
31.08 ±3.68 52.79 ±1.69 [89.9]
35.88 ±0.67 63.98 ±2.21 [49.1]
25.96 ±2.48 50.27 ±0.98 [98.9]
31.37 ±3.70 45.33 ±3.10
4 30
100
47.69 ±1.59 63.12 ±1.04 [25.8]
–
45.89 ±2.29
37.22 ±1.80 67.32 ±0.47 [48.5]
56.4 ±4.79 90.84 ±3.54 [22.1]
42.25 ±2.60 95.78 ±1.68 [31.3]
27.45 ±0.77 83.86 ±1.48 [44.3]
38.34 ±3.93 90.75 ±1.80 [34.4]
5 30
100
47.13 ±2.30 92.74 ±1.22 [29.4]
–
82.31 ±2.64 [89.7]
38.15 ±1.42 94.04 ±0.71 [35.9]
37.91 ±0.69 97.35 ±0.26 [35.2]
47.84 ±1.29 99.42 ±0.13 [25.6]
33.08 ±1.58 99.00 ±0.23 [37.2]
32.24 ±2.91 92.33 ±1.15 [35.6]
6 30
100
22.82 ±2.60 –
– –
– –
– –
– –
– –
– –
7 30
100
33.08 ±2.58 64.78 ±0.06 [49.8]
–
22.25 ±0.91
24.48 ±2.86 39.41 ±1.68
–
30.85 ±2.23
24.49 ±2.17 44.1 ±2.15
22.00 ±1.45 33.46 ±0.61
30.29 ±1.28 27.96 ±5.50
8 30
100
39.71 ±0.72 67.30 ±0.38 [41.8]
–
25.24 ±1.51
36.21 ±1.69 48.95 ±2.04
36.53 ±2.66 57.40 ±1.03 [64.9]
36.66 ±0.99 57.37 ±1.91 [57.4]
–
43.68 ±1.80
25.35 ±0.44 40.42 ±0.34
9 30
100 34.19 ±0.73
69.06 ±0.72 [50.8]
–
31.69 ±2.74 27.85 ±2.69 57.93 ±3.04 [61.1]
–
59.59 ±1.47 [81.1]
–
55.56 ±1.04 [89.5]
29.49 ±2.28 47.62 ±1.04
–
49.21 ±2.50
10 30
100
48.19 ±0.62 72.62 ±0.14 [27.9]
26.57 ±1.78 27.94 ±0.95
25.93 ±1.66 50.09 ±2.65
42.27 ±0.66 54.50 ±0.13 [63.8]
27.44 ±2.26 50.98 ±1.45 [98.4]
25.86 ±1.90 53.07 ±2.30 [93.8]
28.19 ±2.98 42.27 ±1.93
11 30
100 74.48 ±1.01
83.80 ±0.33 [6.0]
55.97 ±2.09 55.24 ±0.87 [25.9]
61.19 ±0.39 78.37 ±0.20 [16.9]
43.03 ±1.31 75.87 ±1.40 [36.4]
76.71 ±0.16 83.69 ±0.69 [8.3]
68.96 ±0.41 70.42 ±0.94 [7.9]
46.00 ±3.22 53.89 ±1.47 [47.1]
12 30
100
59.48 ±0.53 68.75 ±1.06 [51.3]
–
57.66 ±1.49 [86.3]
53.17 ±4.07 98.52 ±0.55 [21.7]
20.79 ±2.39 97.48 ±0.26 [42.5]
–
68.78 ±1.88 [63.4]
21.80 ±0.85 79.58 ±2.00 [43.1]
26.70 ±0.45 76.51 ±1.03 [46.8]
13 30
100
–
46.58 ±0.94 –
34.35 ±1.99 –
26.24 ±0.04 – –
–
34.48 ±4.04 –
27.82 ±1.60
30.37 ±2.68 29.02 ±0.54
14 30
100 33.23 ±0.97
– –
–
22.11 ±2.66 – –
–
24.31 ±2.31 – –
– –
15 30
100
–
32.37 ±1.21 – –
– –
– –
– –
–
26.06 ±1.51
28.00 ±1.03 20.32 ±2.53
16 30
100
–
28.95 ±1.00
27.66 ±0.65 36.30 ±1.49
– –
– –
–
30.90 ±0.52 –
28.86 ±0.02 –
29.77 ±3.55
17 30
100
– –
– –
– –
– –
– –
– –
– –
18 30
100
–
48.01 ±4.32 – –
– –
– –
– –
– –
– –
19 30
100
69.04 ±1.32 81.39 ±1.77 [7.7]
–
43.5 ±1.39
47.80 ±1.91 74.88 ±2.14 [24.5]
45.26 ±0.86 63.47 ±0.37 [47.5]
56.67 ±2.59 69.90 ±2.73 [27.0]
50.17 ±0.96 72.99 ±0.07 [33.5]
31.43 ±2.26 50.00 ±1.96 [92.5]
20 30
100 23.36 ±1.74
51.91 ±0.12 [79.8]
– –
– –
– –
– –
– –
– –
21 30
100
–
20.84 ±0.12 – –
– –
– –
26.36 ±0.22 39.88 ±3.01
24.60 ±1.66 33.86 ±0.65
– –
22 30
100
–
37.98 ±0.47 – –
– –
– –
34.13 ±3.27 34.94 ±0.75
– –
– –
23 30
100
– –
–
23.09 ±1.22 – –
– –
– –
20.72 ±0.67 33.90 ±0.62
– –
( continued on next page )
Table 9 ( continued )
Comp. Conc.( μM) Growth inhibition;% ±SEM [calculated IC 50value; μM] ¥
HeLa SiHa C33A A2780 MCF-7 T47D MDA-MB-231
24 30
100
24.46 ±5.28 72.98 ±2.97 [57.3]
– –
–
35.50 ±0.30 –
28.94 ±1.45 –
28.30 ±1.11 –
32.36 ±2.44 – –
5-FU 10
30 22.69 ±0.89
25.60 ±0.79 [n.e.] §
40.98 ±0.98 57.07 ±1.18 [19.2]
35.18 ±1.36 49.97 ±1.25 [24.8]
62.22 ±2.35 69.06 ±1.83 [8.5]
30.47 ±1.38 43.84 ±0.85 [42.9]
43.37 ±0.91 52.54 ±0.72 [22.9]
45.82 ±3.09 59.72 ±1.36 [13.7]
¥ Mean value from two independent measurements with five parallel wells; standard deviation < 20%.
∗ Growth inhibition values ˂20% are not presented.
#IC 50values have been calculated if the growth inhibition value of the compound at 100 μM concentration is higher than 50%.
§n.e. = not effective, the calculated IC 50value of 5-FU is higher than 100 μM.
║%INH values.
benzyl group as R2 and R3 substituents (Table 9). If the 3,4- dimethoxyphenyl fragment changed with p-chlorophenyl modest antiproliferative activity selective to HeLa cell line was obtained (compound24).
AnothergroupofBiginelliderivativescanbecreatedfromcom- pounds4,5,and21becausetheseanalogsdifferfromeachother inthe lengthofthehalogenatedalkylchainatpositionR2 ofthe phenylring only.21 containsahydroxyl group asR2substituent, therefore it is the basic derivative of thisgroup. The mentioned compounddidnotexertasubstantialcellgrowthinhibitoryeffect against thetestedcancercell lines,becauseitwasableto inhibit cell proliferationatamaximumof40%(Table9).After furnishing halogenated alkyl chains(i.e.bromobutoxy orbromopentoxy) the antiproliferative activitysubstantially increasedon all testedcan- cercelllinescompared to21.However,thereisnosifnificantdif- ference between theIC50 valuesof4 and5 excepton SiHacells, thereforethelengthofthechainhasnoimpactonthepharmaco- logicaleffectinthiscase. OnemoreBr-containingBiginellideriva- tive hasbeentested, compound12,whichpossesses methylester fragment atposition R1,unlike the other membersofthisgroup.
Itsantiproliferativecapacitycanbecomparedtothatof8because their chemical structurediffersinone Br-atomonly.Theincorpo- rationofthehalogenatomincreasedthecellgrowthinhibitorypo- tentialof12,exceptonHeLa cells,however,thismodificationled toamedium-effectiveTHPM(Table9).
Theinfluenceofthestructureofthefunctiongroupatposition R1 canalsobe thebasis ofacomparisonamongthetestedTHPM analogs.Compounds3and10possessasimilarchemicalstructure with a long, branched-chain substituent withdouble C–C bound asR2functiongroup,however,theyaremethylesterandethyles- terderivatives,respectively,regardingpositionR1.Thelongerfunc- tion group at position R1 slightly increased the antiproliferative activity of 3. A very similar trend can be observed in the case of cell growthinhibitory potential ofcompounds 2 and9,which contain O-butenyl group at position R2 (Table 9). Compound 13 hasO-allylfunction.Thisslightstructuralmodificationresultedin thedecreasedantiproliferativeactivityof13comparedto9.More- over,compounds8and13containthecorrespondingO-propyland O-propenyl (allyl) function, respectively. The presence of unsatu- rated bondreducedantiproliferativeactivity of13compared to8 (Table9).
ThefollowingthreeBiginellihybridspossessacetylgroupasR1 substituent(6,17,and20),however,theyhavevarioussubstituents on THPMs core. Presumably,dueto the presenceof acetylgroup thesemoleculesdidnot exertanysubstantialantiproliferativeac- tivityagainstthetestedcancercelllinesexceptof20whichinhib- itedHeLacellproliferationwithIC50 valueof79.8μM(Table9).
Two 2-thioxo analogs, compounds 1 and 7 have been tested against gynecologicalcancer cell lines. Although they differ from each other in thesubstitution patternof phenylring,it doesnot
haveasignificantimpactontheirantiproliferativecapacities:both compoundswereabletoinhibitHeLa cellproliferationwithmod- estIC50 values(99.1μMand49.8μM,respectively)buttheycould not substantially influence cell growthof other examined cancer celllines(Table9).
Insummary,twocompounds(11and19)amongoftwenty-four investigatedTHPMs havebeenidentified aspromisingcell prolif- erationinhibitingagentsonHeLacellline.Additionally,compound 11wasabletosuppressproliferationofMCF-7andT47Dcelllines withlowIC50values.Theirantiproliferativeactivitiesagainstthese cancercell linesexceedthat ofourpositivecontrol, 5-FUwithat leastoneorderofmagnitude.Moreover,tumor-selectivityofthese two effective compounds has also been examined by means of MTT-assayagainst mouseembryo fibroblast cells. The IC50 values of11and19onfibroblastcellsare92.3and31.1μM,respectively.
Highselectivityindexof11and19(15.4and4.0,respectively)in- dicatethat theircellproliferationinhibitoryactivities canbecon- sideredasselectivetothetumorcells(Table10).
3. Materialandmethods
Biginellihybridsweresynthesizedusingearlierdescribedmeth- ods [13,14]. Methanol and acetonitrile (HPLC grade) were pur- chasedbySigma.
3.1. ChromatographicbehaviorofselectedBiginellihybrids
In this work, twenty-fourBiginelli hybrids were exploited for the establishment of their molecular properties. For the evalua- tionofretentionparametersRPTLCanalysiswasemployed.Inthis analysis solutions of analytes were prepared in concentration of 1 mg/mL.Analytes were applied on the RP TLC plateas individ- ual bands, and the plates were developed using MeOH/H2Oand MeCN/H2O solvent systems with (MeCN/MeOH) = 0.5–0.7 v/v.
Inexperiments,1
μ
L aliquotof eachanalyte solution wasspottedonthe plateusing amicropipette.Plates are thendeveloped ina chamber tank(Camag) whichwassaturatedwiththeappropriate eluentovernightat25°C.TheexperimentwasperformedonRP-18 modifiedsilicagelF254s(20×20cm)plates(MerckDarmstadt).
ThespotsofanalytesarevisualizedusingUVlampat254nm.Each RP-TLCanalysiswasperformedintriplicateunderequalconditions oftemperatureandhumidity. Nosignificant statisticaldifferences oftheretentionfactor(Rf)wereobserved.Theretentionconstants (RM)werecalculatedusingtheequation:
RM=log
(
1/Rf−1)
RfrepresentsretentionfactorandRMvalueslinearlydependonthe logarithm of concentration organic modifier in the mobile phase accordingtoSoczewinski[19]
RM=R0M+blogC