with an accelerated decline?
Brigitta Kakuszi, Bálint Szuromi, István Bitter, Pál Czobor
∗SemmelweisUniversity,DepartmentofPsychiatryandPsychotherapy,Balassau.6.,Budapest1083, Hungary
Received 24September2019;receivedinrevisedform9March2020;accepted13March2020
KEYWORDS ADHD;
Neuro-development;
Maturational-delay;
NoGoP3;
EEG;
Age-relateddecline
Abstract
WhileearlyneurodevelopmentalprocessesduringtheemergenceofADHDinchildhoodreceived considerableattention,theneurobiologicalmechanismsthatunderliethechangesinADHDin adulthoodremainlargelyunaddressed.Wewantedtodelineateneurodevelopmentalchanges inadultADHDusinganelectrophysiologicalmeasure,thefronto-centralNoGoP3event-related potential(ERP),whichisanimportantneurophysiologicalindexofbrainfunctioninginADHD, andbiomarkerfor responseinhibitionandaging.ERPs wereobtained from45ADHD and41 healthysubjectsusinga128-channelBioSemirecording-system,applyingemotionally-valenced andneutral stimuliin aresponse inhibition task. Our resultsindicated that ADHD subjects manifesteddelayeddevelopmentalP3-trajectoryinyoung-adulthoodascomparedtocontrols;
theyalsoshowedP3reductionacrossallemotionalvalences,andthereductionwasmostpro- nouncedatyoungerages.ThedifferencesinP3diminishedbymid-adulthood,andstartedto increaseagainatmoreadvancedages.Thus,similartostructural-MRIindices,developmental braindifferencesinthefronto-centralNoGoP3inADHDlargelynormalizeinyoung-adulthood.
However,areductionofP3occursagain startingfrommid-adulthood.Asthefronto-central NoGoP3reflectsthefunctioningofthefrontalareas(whichshowdelayedmaturationinADHD), ourfindingsareconsistentwiththe‘‘lastin,firstout’’hypothesis,whichreferstoamirroring patternofbraindevelopmentandaging,andpositsthatbrainregionsthatdeveloprelatively latedegeneraterelativelyearlywithage.Thus,ADHDmaynotonlybeassociatedwithdelayed neurodevelopment,butalsowithaprematureage-relateddeterioration,atleastinsomemea- suresofelectrophysiologicalfunctioning.
©2020TheAuthors.PublishedbyElsevierB.V.
ThisisanopenaccessarticleundertheCCBY-NC-NDlicense.
(http://creativecommons.org/licenses/by-nc-nd/4.0/)
∗Correspondingauthor.
E-mailaddress:czobor.pal@med.semmelweis-univ.hu (P.Czobor).
https://doi.org/10.1016/j.euroneuro.2020.03.011
0924-977X/©2020TheAuthors.PublishedbyElsevierB.V.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense.
(http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
While early neurodevelopmental alterations during the emergenceofADHDinchildhoodreceivedconsiderableat- tentionintheliterature(Shawetal.,2006;Spronketal., 2008; Vaidya,2012),the neurobiological mechanismsthat underliethe changesinADHD inadulthood remainlargely unaddressed. With respect to early neurodevelopmental processes, longitudinal structural-MRI data indicate that children withADHD undergo delayed maturation in many brainregions,especiallyinthefrontalcortex(Gogtayetal., 2002;Shawetal.,2007).Asignificant portionofthismat- urational delay, however, is being made up by thebegin- ningofyoung-adulthood(Shawetal.,2007).Alargecross- sectional investigation of structural-MRI data - termed as
’mega-analysis’bytheauthors(Hoogmanetal.,2017)-of 1723ADHDand1523controlsubjects(agerange=4–63years) providedstrongsupportforthe"brainmaturationdelaythe- oryfor ADHD".Sinceitis conceivablethat -throughbrain plasticityorexperience-dependentsynapticreinforcement (Caseyetal.,2005;Whitfordetal.,2007)-thesechanges in brainmorphology duringchildhoodand adolescencein- ducefunctionalalterations,itwouldbeimportanttoinves- tigatepost-adolescentneurophysiologicalchangesinADHD as compared to healthy individuals. In terms of func- tionalbrainalterations,arecentlypublishedcomprehensive overview of developmentaltrajectories of ADHD over the lifespan(Frankeetal.,2018)identifiedonesmall,longitu- dinalEEGstudy(Doehnertetal.,2013)whichreportedthat onlyERPimpairmentsrelatedtoresponsepreparationwere associatedwithADHDinadulthood.However,thestudywas small(ADHDn=11/controln=12)andthefollow-upofsub- jects(childrenwithameanage=10.9years)didnotextend beyondyoungadulthood(meanage=21.9years).
An importantelectrophysiologicalmarkerof brainfunc- tioning in ADHD is the fronto-central P3 ERP, a positive- going potential between approximately 300 to 450 msec post-stimulus,which iswidelyviewedasameasureofbe- havioral response inhibition (BRI) (Fallgatter etal., 2005; Knezevicand Marinkovic,2017;RandallandSmith, 2011), andof aging(Rossinietal.,2007). Itistypicallyobserved afterNoGotrialsinresponseinhibitionparadigms,andmay reflecttheactivityofmultiplecorticallocations,including thefrontalcortex,pre-supplementarymotorarea,thecin- gulateregionsandtheinferiorparietallobe(Albertetal., 2013; Tammetal., 2002;Vara etal.,2014).BRI is acore executive function (EF); its impairmentleads tobehavior dyscontrol, distractibility and deficits in sustained atten- tion, which are considered to be key features of ADHD (Wodka etal., 2007).Diminished ability tosuppress inap- propriateprepotentresponsestostimulihasbeenobserved inADHD(LipszycandSchachar,2010;Wodkaetal.,2007), which hasbeen viewedbysome authors asa "disorderof deficient self-regulation"(DSR) (Barkley, 1997). BRI under- goesa prolonged maturation:in typicallydeveloping (TD) subjects,itsdevelopmentstartsattheageof6years,and lasts at least until the age of 20 years (Jonkman, 2006; Tammetal.,2002).
In line withthe developmental changesof BRI, P3also shows a protracted developmentalpath in TDchildren. It cannot yet be observed in 6–7-year oldchildren (while it is present in 9–10 year olds), which hasbeen interpreted
as a sign of immature response inhibition processing in earlychildhood(Jonkman,2006).Anotherstudyalsofound thatthe NoGo P3develops relatively late,around age 10 (Okazakietal.,2004).AsystematicreviewbyvanDinteren etal.2014identifiedaquadratictrajectoryfromchildhood tooldagesforthefronto-centralP3amplitude:asteadyin- creasefrompreschool-ageuntilamaximum ataroundthe third-decade;andasubsequentplateau,followedbyade- clinefromthefifth-decade.
Astwoimportantsourcesofthefronto-centralP3arethe frontal cortexand the cingulateareas, and its amplitude correlateswiththefrontalgraymattervolume(Fordetal., 2004), a parallel can be drawn between the gradual in- crease of P3 amplitude with age and the prolonged de- velopment of the frontal cortex. Importantly, the NoGo P3, as a neural marker of BRI, exhibits altered develop- mentinADHD:diminishedNoGoP3isconsistentlyreported in ADHD as compared to TD children between 7 and 14- yearsofage(Fallgatteretal.,2004;Fallgatteretal.,2005; Wiersemaetal.,2006).
Prior studies of NoGo P3 ERP almost exclusively used BRI tasks which ignored affective contexts, and did not examinewhetherBRIisaffectedbythestimuli’semotional valence(see,e.g.,Albertetal.foranexceptioninhealthy subjects(Albertetal.,2013)).ThesestudiesconsideredEFs aspurelycognitive skills(Tsermentseli andPoland, 2016).
However, more recent theoretical models of EFs make a distinction between cool cognitive EFs (i.e., primarily thoseencompassed in the earlier conceptualization, such as attention, working memory, planning and inhibition) and “hot” EFs which involve emotions and motivation (ZelazoandCarlson,2012).Thistheoreticalframeworkhas crucialimplicationsforthedevelopmentalresearchinADHD becausecurrentconceptualizationsconsiderDeficientEmo- tional Self-Regulation (DESR) as a core feature of ADHD (Barkley, 1997; Castellanos et al., 2006; Surman et al., 2013).Nonetheless,wearenotawareofanydevelopmen- taldataontheP3ERPinADHDduringadulthoodthatwere obtainedinanemotionalresponseinhibitiontask.
Tofillintheaboveknowledgegaps,inthecurrentstudy wefocusedonthefronto-centralP3ERPtoinvestigatethe neurobiologicalunderpinningsofdevelopmentalchangesin ADHDduringadulthood.Usingaquadraticregressionmodel- ingapproach,wewanted(a)todelineatethedevelopmen- tal trajectory of P3ERPin ADHD ascomparedto healthy controls; and (b) to examine whether the developmental changes are associated with changes in response inhibi- tion.Weadoptedanemotionalresponseinhibitionparadigm basedona Go/NoGo task usingemotionally-valenced and neutral pictures from the International Affective Picture System(IAPS)(Langetal.,2008).
2. Experimentalprocedures 2.1. Studysample
Patients who met DSM-IV criteria for adult ADHD (mean age=30.4years, range:18–59years) and had no history of neuro- logicalillnesswererecruitedforthestudyattheDepartmentof Psychiatryand Psychotherapy, Semmelweis University,Budapest, Hungary,whichprovidesoutpatientserviceforpatientswithadult
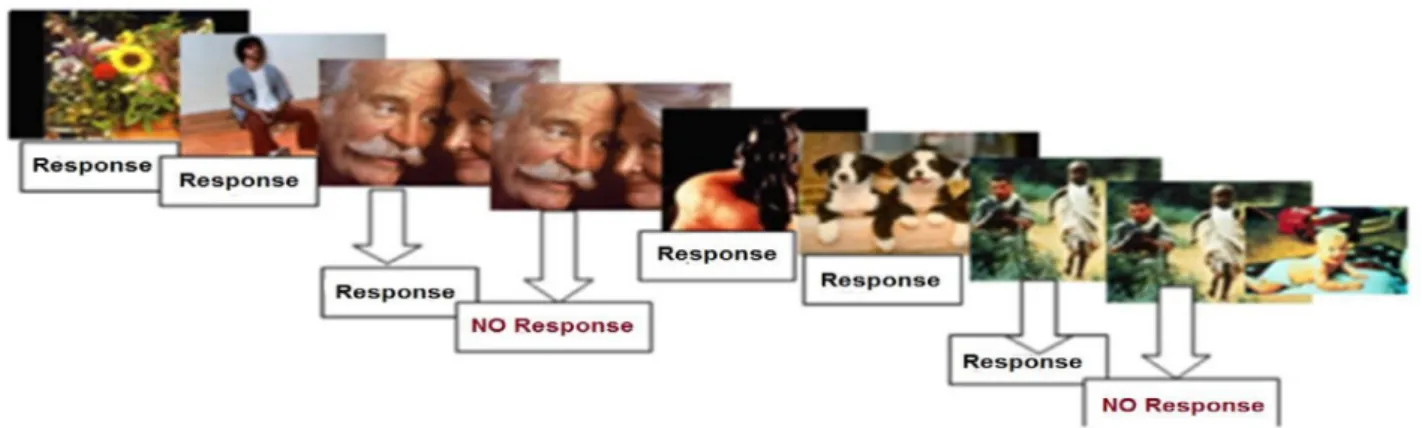
Fig.1 Taskandprocedure.Stimuliwerepresentedcentrallyevery1400msecfor800msec(inter-stimulus-interval=600msec).
Subjects wereaskedtopushabuttonassoonaspossibleupontheappearanceofthestimuluspictures(Gotrials);they were, however, askedtowithholdresponse incase apicturewasrepeated(NoGotrials). 240stimuliwere shownintwo blocks.The probabilityofGoandNoGotrialswas0.85and0.15,respectively.
ADHD. All included patients fulfilledthe DSM-IV criteriafor the combinedsubtypeofADHD(i.e.,had>6symptomsofthetotalof 9 symptomsofInattentionand Hyperactivity/Impulsivity,respec- tively). Recruitment was done bystaff of the outpatientclinic.
Diagnosiswasconfirmedthroughsemi-structuredinterviewbythe treatingphysician.Healthycontrols(HC)wererecruitedfromthe community throughfriendsandacquaintancesofthestaff ofthe University.HCshad amean ageof31.3years(range:18–59years), and had no history of psychiatric disease or currentpsychiatric comorbidity. They wereindividuallymatchedto patientson age (±5years),genderandeducationlevel.Additionalexclusioncrite- riaforparticipantsinthecontrolgroupwereanypresentorpast neurologicaldisorderandhistoryofheadinjurywithlossofcon- sciousness.Thestudycompliedwiththeethicalstandardsofthe DeclarationofHelsinki,andreceivedapprovalfromthelocalEthi- calCommittee.AllparticipantsgavewrittenInformedConsentfor thestudy.
2.2. Descriptionofmeasures
The Conners’ Adult ADHD Rating Scale (CAARS;66-item version) was used to characterize ADHD symptom severity across core psychopathological domains of ADHD: Inattention, Hyperactiv- ity, Impulsivity and Problems with Self-Concept(Conners, 1999; Erhardtetal.,1999).TheAdultSelf-ReportScalesymptomCheck- list(Adleretal.,2006)wasusedtodelineateADHDsymptomsand toestablishADHDsubtype.ThetotalscoreontheSymptomCheck List 90R(SCL-90R), a self-report scale was used to measure the severityongeneraldomainsofpsychopathology(Derogatis,1994).
Thescalewasdesignedprimarilytoassesssymptompatternsina broadspectrumofpopulations,ranging fromnon-patienthealthy subjects to individuals with psychiatric disorders(Derogatis and Cleary, 1977). The scale was administered by study person- nel including psychologists and researchassistants trained inits administration.
2.3. Stimuliandprocedure
Participantswereseatedinadimlylitroom,approximately100cm fromthemonitor.TheInternationalAffectivePictureSystem(IAPS) (Langetal.,2008),asetofimageswithneutral,positive,andneg- ativevalences,wereusedasstimuli,andpresentedinrandomse- quenceusingthePresentation13.0software(NeurobehavioralSys-
tems, Inc.,Albany,Calif.).The stimuliwerepresentedcentrally every1400msecfor800msec(interstimulus-interval=600msec).
Atotalof480stimuliwerepresentedintwoblocks,eachconsisting of240stimuli.Subjectswereaskedtopushabuttonassoonaspos- sibleuponappearanceofthepictures(Gotrials);theywereasked towithholdresponseifapicturewasrepeated(NoGotrials).The probabilityofGoandNoGotrialswas0.85and0.15,respectively.
ThestimulustaskandprocedureareillustratedinFig.1.
2.4. EEGrecordingandpre-processing
High-densityEEGswererecordedusinga128-channelBioSemiAc- tiveTwosystemwithanaveragereference,atadigitizationrateof 1024Hz,applyingaband-passfilterof0.5–70Hz.Weappliedastan- dardBioSemi128-electrodeheadcapsystem(onlinesource:http://
www.biosemi.com/pics/cap_128_layout_medium.jpg). Data were storedandanalyzedoff-lineusingtheElectromagneticSourceSig- nal Imaging (EMSE)Suite as well as theStatistical Analysis Sys- tem (SAS 9.4) software. EEG was re-referenced off-line to the common average potential and filtered between 0.5 and 70Hz using zero-phase shiftforward and reverse IIRButterworth-filter.
Additionally, the signal was filtered using the 48–52Hz Parks- McClellan stop-band Notch filter. The notch filter was used to remove any potential electric-interference from the 50Hz line.
Artifacts dueto blinks and eyemovements were removedman- ually and with the electrooculography artifact removal proce- dure. Epoch selection for the analyses was conducted manu- ally, as well as applying automatic artifact rejection criteria.
Stimulus-lockeddataweresegmented intoepochsof1000msec, including200msecbeforestimulus and800msecafterstimulus.
Segmentswithactivityexceeding±100μVwereexcludedfromfur- theranalysis.Thethresholdcut-off was50fortherequiredmini- mumnumberofusablesegmentsfortheERPanalyses.Onlycorrect trialswereincludedinthecurrentanalyses.Thestimulus-locked segmentswerebaseline-correctedusinga200msecpre-response window,andaveragedtoobtaintheERP-waveformsforeachsub- jectandeachemotionalvalence.
2.5. Statisticalanalyses
2.5.1. Behavioraldata
WeusedAnalysisofCovariancewithrepeated-measures(rANCOVA) to investigate behavioral data, including commission-error rates
Table1 Basicdescriptivestatisticsofthestudypopulation.
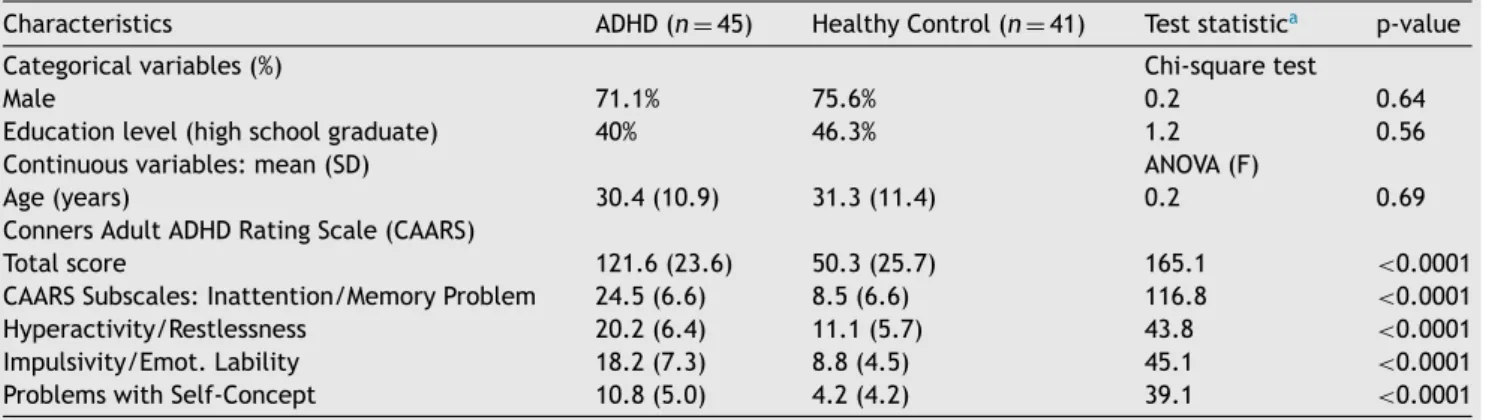
Characteristics ADHD(n=45) HealthyControl(n=41) Teststatistica p-value
Categoricalvariables(%) Chi-squaretest
Male 71.1% 75.6% 0.2 0.64
Educationlevel(highschoolgraduate) 40% 46.3% 1.2 0.56
Continuousvariables:mean(SD) ANOVA(F)
Age(years) 30.4(10.9) 31.3(11.4) 0.2 0.69
ConnersAdultADHDRatingScale(CAARS)
Totalscore 121.6(23.6) 50.3(25.7) 165.1 <0.0001
CAARSSubscales:Inattention/MemoryProblem 24.5(6.6) 8.5(6.6) 116.8 <0.0001
Hyperactivity/Restlessness 20.2(6.4) 11.1(5.7) 43.8 <0.0001
Impulsivity/Emot.Lability 18.2(7.3) 8.8(4.5) 45.1 <0.0001
ProblemswithSelf-Concept 10.8(5.0) 4.2(4.2) 39.1 <0.0001
aANOVAforcontinuousvariables,Chi2−testforcategoricalvariables.
and reactiontimes.Commission-errorrate(%) andreaction time (RT, msec)wereappliedinseparate analysesasdependent vari- ables. Studygroup (ADHD, HC; used asa between-subjects fac- torandemotionalvalence(positive,negative,neutral;,usedasa within-subjectfactor)weretheindependentvariables.Agewasap- pliedasacontinuousregressor.Inordertoexaminepotentialnon- linearchangesovertime,agewasincludedintheanalysisbothas a linearanda quadraticterm.Interactionbetween studygroup, emotionalvalenceandagewerealsotested.
2.5.2. ERPmeasures
Based on literature, the definition of frontal P3 compo- nent time-window included the post-stimulus epoch of 300–
450 msec (Knezevic and Marinkovic, 2017). Similar to our ear- lier work (DeSanctis et al., 2013), thestatistical analyses were based on random-regression hierarchical linear modeling (HLM;
(BrykA.S.andRaudenbushS.W.,1992;Gibbonsetal.,1988)).Re- peatedmeasurementsoftheERPamplitude(inmicrovolts)inthe frontalregions fortheP3 ERP(regionof interestincludedelec- trodeFzandtheadjacentelectrodessurroundingFz)inthecom- ponent time-window served as dependent variable.Study group (between-subjectsfactor),andemotionalvalence(within-subject factor)weretheprincipalindependentvariablesofinterest.Time (samplingpointinthecomponentwindow,relativetostimuluson- set)wasalsoincludedintheanalysisasawithin-subjectfactor.A first-orderautoregressivemovingaveragecorrelationmatrixamong thesamplingpointswasspecifiedintheHLMmodel.Genderserved asacovariate.Similartotheanalysesofbehavioraldata,agewas usedasa continuousregressor.Interactionbetweenstudygroup, emotionalvalenceandage(usedinlinearandquadraticform)was investigatedintheHLM.Theinteractioneffectbetweengroupand agetestedwhetherthestudygroupshadadifferentdevelopmental trajectorywithage.
3. Results
3.1. Demographiccharacteristics
PatientswithADHD(N=45)andHCs(N=41)hadnosignif- icant differencein age(Table1).The proportionofmales wassomewhathigher(75.6%)amongtheHCthanamongthe ADHDsubjects(71.1%); thedifferencewasnotsignificant.
Therewasnosignificantdifferenceintheeducationlevel, with morethan 40% of the sample in both groups attain- ingcollegeorhighereducation.Thetwogroupssignificantly differedonCAARSpsychopathologicalsymptomdimensions:
asexpected,patientshadhigherseverityonallsymptomdi- mensions.ThepatientsamplemetthecriteriafortheADHD combinedsubtype.
Amongthe ADHDpatients, 12(26.6%)of the45patients had comorbidity according to DSM-IV. In the majority of cases(10[22.2%]of45),comorbiditiesfellin theaffective categories.Specifically,affective comorbiditiesincluded8 cases withdepression in their medical history, while dys- thymiaandbipolardisorderoccurredin 1patient,respec- tively.Additionalcomorbiditieswereobsessive-compulsive disorder in one patient, and substance (cannabis) use in onepatient.Approximatelyhalfofthe45patients(n=23, 51.1%)receivedstimulantmedication.Patientstakingstim- ulanttreatmentwereoff medicationforatleast24hbefore testing.
3.2. Behavioralmeasures
Withrespect to commission-errors, theanalysis indicated asignificant maineffectof group(F1,85=4.72,p=0.032), and a significant linear (but no quadratic) effect of age on the commission-error rates (F1,85=8.75, p=0.0041).
Post-hoc analyses showed that ADHD patients made more commission-errorsthanHCs,andthatthecommission-error ratedecreasedwithage.Themaineffectofemotionalva- lence,anditsinteractionwithgroupandagewerenotsig- nificant (p > 0.1 for all respectiveterms in therANCOVA analyses). The developmental trajectory of commission- errorrates,determinedonthebasisofthelinearregression estimates,isshowninFig.2(top).To delineatetheabove resultsintermsoftheobserveddata,wedichotomizedthe studygroupsintoyoung-adult(≤30years)andmiddle-aged (>30years)groups,andprovidedsummarystatisticsforboth agegroups (Table 2,left). In the ADHDgroup, amongthe middle-agedsubjects theproportion of commission-errors wassignificantly(p<0.05)reducedforallthreeemotional valencesascomparedtotheyoungersubjects.Among the HCs,thereductionwassignificantonlyfortheneutralstim- uli.
The analysisof RTs showed a linearmain effect of age (F1,85 = 13.7, p=0.0004), with no quadratic or interac- tioneffects.Post-hocanalysesindicatedthatinbothstudy groups RTs increased with age. The estimated RT trajec-
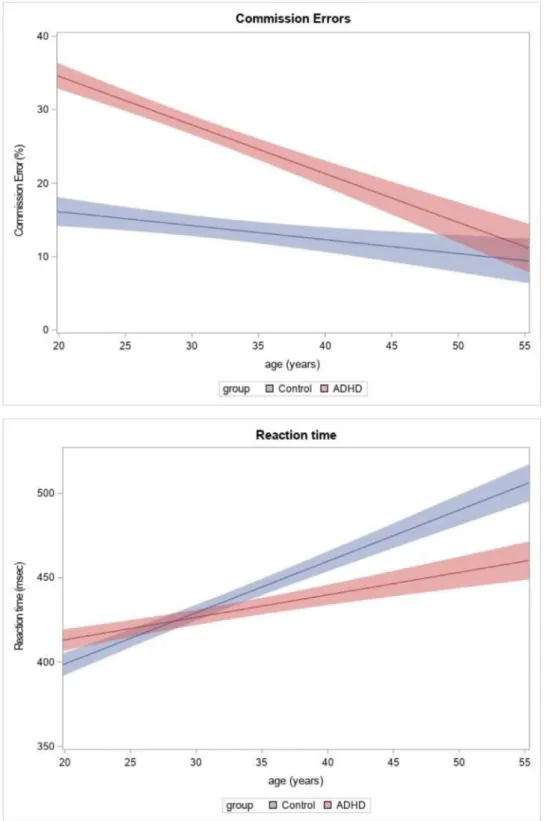
Fig.2 Commission-errorrate(toppanel)andreactiontime(bottompanel)asafunctionofageintheADHDandthehealthycontrol group,determinedonthebasisofthelinearregressionestimatesobtainedfromtherepeatedmeasuresANCOVAmodel.Theshaded bands depictthestandarderroroftheestimates.For commission-errors,theanalysis indicatedasignificant (p=0.032)global effectofgroup,withahighererrorrateamongADHD subjectsirrespectiveofemotionalvalence;andasignificant(p=0.0041) reductionoferrorratewithage.Themaineffectofemotionalvalence,anditsinteractionwithgroupandagewerenotsignificant.
Forreactiontime,theanalysisshowedasignificant(p=0.0004)linearagemaineffect,indicatingincreasedreactiontimeswith ageinbothgroups.Thedifferenceinreactiontimebetweenthetwogroupswasnotsignificant,andtheage-relatedchangesdid notshowasignificantinteractionwithemotionalvalenceorstudygroup.
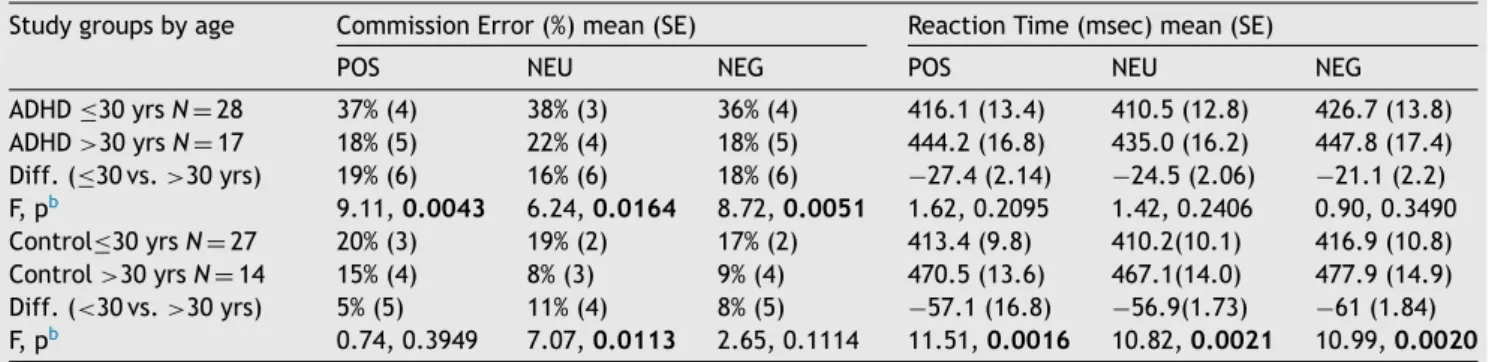
Table2 CommissionerrorratesandreactiontimesbyagegroupamongADHDandhealthycontrol(HC)subjectsa. Studygroupsbyage CommissionError(%)mean(SE) ReactionTime(msec)mean(SE)
POS NEU NEG POS NEU NEG
ADHD≤30yrsN=28 37%(4) 38%(3) 36%(4) 416.1(13.4) 410.5(12.8) 426.7(13.8) ADHD>30yrsN=17 18%(5) 22%(4) 18%(5) 444.2(16.8) 435.0(16.2) 447.8(17.4) Diff.(≤30vs.>30yrs) 19%(6) 16%(6) 18%(6) −27.4(2.14) −24.5(2.06) −21.1(2.2) F,pb 9.11,0.0043 6.24,0.0164 8.72,0.0051 1.62,0.2095 1.42,0.2406 0.90,0.3490 Control≤30yrsN=27 20%(3) 19%(2) 17%(2) 413.4(9.8) 410.2(10.1) 416.9(10.8) Control>30yrsN=14 15%(4) 8%(3) 9%(4) 470.5(13.6) 467.1(14.0) 477.9(14.9) Diff.(<30vs.>30yrs) 5%(5) 11%(4) 8%(5) −57.1(16.8) −56.9(1.73) −61(1.84) F,pb 0.74,0.3949 7.07,0.0113 2.65,0.1114 11.51,0.0016 10.82,0.0021 10.99,0.0020
aStudygroups(ADHD,HC)weredichotomizedbyage(≤30years[youngadults]vs.>30years[middle-agedsubjects]).
bANOVAwasappliedforbothstudygroupstoexaminedifferencesincommissionerrorsratesandreactiontimesbetweenthetwoage groups(youngadultsvs.middleagedsubjects).Theanalyseswereconductedseparatelyforthepositive,neutralandnegativestimuli.
Errorrate(%)andreactiontimewasappliedasdependentvariable.Agegroup(≤30yearsvs.>30years)wasusedasanindependent variable.Significant(p<0.05)differencesbetweenagegroupsarehighlighted.
Fig.3 GroupaveragedNoGoERPresponsestonegative,neutralandpositivepicturesintheemotionalNoGoparadigm,broken downbystudygroup(ADHD,Control).ShadedareasindicatethetimewindowofinterestfortheP3ERPcomponent.Asshownbythe Figure,theP3componentwasprecededbyalargenegative–goingwave,thefrontalN2,whichshiftedthecomponentdownward (i.e., innegativedirection).Random-regression Hierarchicallinear model (HLM)analyses showed asignificantly diminished P3 amplitudeintheADHDgroupforeachofthethreeemotionalvalences(negativep=0.0043;neutralp=0.038;positivep=0.023).
tory with age, determined on the basis of the regres- sion estimates, is shown in Fig.2(bottom). Similartothe commission-errors, we alsocharacterized the abovefind- ings in terms of the observed data by subdividing the study groups intoyoung-adult andmiddle-aged subgroups (Table 2, right). By mid-adulthood, the RTs increased nu- mericallyinboth theADHDandtheHCgroupfor allemo- tionalvalences.Theincrease,however,obtainedstatistical significanceonlyfortheHCs.
3.3. P3amplitude:groupdifferenceand developmentaltrajectories
Group-averaged NoGo ERPstonegative, neutral andposi- tivepicturesareshown inFig.3separatelyfor bothstudy groups.ThetimewindowofinterestfortheP3component is marked asa shaded area.As shown by the figure, the P3component wasshiftedin negativedirection by apre- cedinglarge negative–goingwave,thefrontalN2,whichis typicallyenhanced invisual tasks(Folsteinand Van Petten C.,2008;Zordanetal.,2008),especiallyunderexperimen-
tal conditions that involve considerable conflict process- ingeffort(Donkers andvan Boxtel, 2004; Gajewski et al., 2008; Nieuwenhuis et al., 2003). HLM analysis indicated a main effect of group with a larger P3 among the con- trols(F1,85 =6.15,p=0.015),andaninteraction between groupandemotionalvalence(F1,85= 4.04,p=0.019).The two-way interaction of group with the linear or with the quadraticeffectofageontheP3amplitudewasalsosignif- icant(linearF1,85=5.08,p=0.027,quadraticF1,85=5.24, p=0.0247).The three-wayinteraction ofagebygroup by emotionalvalencewasnotsignificant(p>0.1).
Post-hocanalysis of theinteraction between group and emotional valence showed a significantly lower P3ampli- tudein theADHD groupfor thenegativepicturesascom- paredtothepositive(t=−3.44,df=85,p=0.0009)orneu- tralones (t= −2.40, df=85, p=0.0187). The difference between positive and neutral pictures did not reach sig- nificance (t=−1.62, df=85, p=0.11). In the HC group, therewere nodifferencesby emotional valence (p > 0.1 inallpairwisecomparisons).Post-hocanalysisoftheinter- action of group withthe linear andthe quadratic effects of age revealed that in the ADHD group both the linear
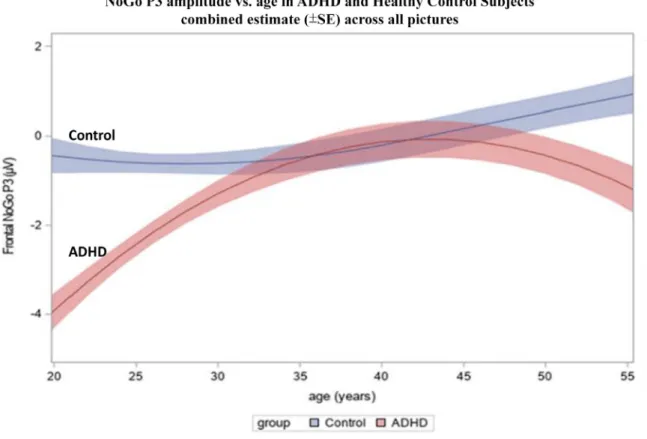
Fig.4 NoGoP3amplitudeasafunctionofageintheADHDandhealthycontrol(HC)groupscombinedforallstimuluspictures.HLM analysisindicatedamaineffectofgroup(F1,85=6.15,p=0.015),andaninteractionofgroupwithboththelinearandthequadratic effectofageontheP3amplitude(linearF1,85=5.08,p=0.027,quadraticF1,85=5.24,p=0.0247).IntheHCgroupneitherthe linearnorthequadraticregressioneffectsobtainedsignificance,indicatingnosystematicchangeinP3amplitudewithage.Inthe ADHD group,boththelinearandthequadraticeffectwassignificant(p<0.05),indicatingacurvilinearrelationship withage.
ThedisplayedtrajectoriesrepresenttheestimatedregressionfunctionsofP3amplitudewithagebasedjointlyonthelinearand quadratictermsfromtheHLM.Theshadedbandsdepictthestandarderroroftheestimates.
andthequadraticeffectsreachedsignificance(lineareffect t=2.47,df=85,p=0.0156);quadraticeffectt=−2.23, df = 85, p=0.0286), indicating a curvilinear relationship of P3 amplitude with age. In the HC group, neither the linear nor the quadratic effectwas significant (p > 0.1), indicating no systematic change in P3 amplitude with age.
Fig.4showstheregressionestimatesfortheNoGoP3am- plitudeasafunctionofage(i.e.,theestimatedquadratic regressionfunctions),combinedacrossallstimuluspictures.
ThedevelopmentalpathoftheP3amplitudewasmarkedly differentinpatientsandcontrols:patientswithADHDevi- dencedasignificantamplitudereductionatyoungeragesas comparedtoHCs.Theamplitudereductiondiminishedand lost significancebymid-adulthood,butthenstarted toin- crease again at higherages.Even though HCs exhibiteda slightincreasewithage,theabovementionedgroupdiffer- enceswereprimarilydrivenbythesubstantiallymorepro- nouncedage-relatedchangesintheADHDgroup.
3.4. Subsidiaryanalyses
In subsidiaryanalyses, we investigatedwhethertheinclu- sion of clinicallyrelevant covariates, suchasthe severity
of psychopathology, comorbidities and medication status, had an impact on our principal electrophysiological find- ings. Fortheseverityof psychopathologywe usedthe to- talscoreontheCAARSscale,whileadichotomousvariable wasapplied for the use of stimulant medication (yes/no) andcomorbidities.OurresultsareshowninOnlineTable1. The rows of the Table depict the results of the addition of the covariates. The table shows that the group ef- fect and the linear and quadratic interaction terms re- mained similarto those obtained from the analyses with no covariate in the model. Specifically, after the intro- duction of the covariates in the analyses, there was no change in termsof statistical significance withrespectto the group effect and the linear and quadratic age inter- actions. Furthermore, the covariate-effects did not ob- tainstatisticalsignificance inthe analyses.We alsoinves- tigated whethertherewasa differencein the severityof psychopathology inthe ADHD sample between the young- adultandmiddle-agedsubgroups.TheANOVAanalysesindi- catednodifferenceintheCAARS totalscore(F1,44=0.12, p=0.73): the mean total score was 120.6(SD=24.1) and 123.2(SD=23.4) in the young-adult and middle-aged sub- group, respectively. Furthermore, the two subgroups did not differ in any of the CAARS subscales (p > 0.1 in all analyses).
4. Discussion
Our principal findings suggest that patients with ADHD manifestadelayeddevelopmentaltrajectoryofthefronto- central NoGo P3 in young-adulthood relative to HCs. In general,thedevelopmentaltrajectoryofP3wasmarkedly differentinthepatientsandcontrols. Whilepatients with ADHD showed a significant P3 reduction across all emo- tionalvalences,thereductionwasthemostpronouncedat youngerages.TheagedifferenceinP3diminishedbymid- adulthood;thisdiminutioneffect,however,waslimitedto a certainage range,beyond which the differencestarted toincreaseagainathigherages.
ConcerningthediminutionofP3alterationinADHDwith age,ourfindingsparalleltheresultsofseveralMRIstudies that examined age-relatedchanges. Forexample, a large longitudinalstudyin223ADHDand223normally-developing children showedthatthepeakof cortical-thicknessmatu- ration was delayed in ADHD by an average of 3 years; in frontalandtemporalareas,thematurationwasdelayedby upto5years(Shawetal.,2007).Arecentlongitudinalstudy bythesameresearch-groupextendedthepreviousfindings by demonstrating that the maturation of cortical surface areawasalsodelayedinchildrenwithADHD(Shawetal., 2012). Dataonpotential developmentalchangesin adult- hood were yielded by two MRI meta-analyses (Frodl and Skokauskas, 2012; Nakao et al., 2011), which included cross-sectional data fromchildren and adults(from sepa- ratestudies).Thesemeta-analysesprovidedconsistentev- idence that the age-related alterations in structural-MRI measures diminish in adult ADHD. A recently published structural-MRI study (Onnink et al., 2014) which exam- ined volumetric measures in a large cross-sectional sam- pleofadultADHDpatientsconcludedthat“developmental braindifferencesin ADHDlargelynormalizein adulthood”
(Onninketal.,2014).
ConsistentwiththeaboveMRIdata,ourresults suggest that a normalization of developmental brain differences in ADHD in adulthood may also occur in terms of neuro- physiologicalmeasures.Asthevolumeofthefrontalcortex wasshowntocorrelatewiththeP3amplitude(Fordetal., 2004),thisfindingisinlinewiththeassumptionofdelayed developmental maturationof thefrontal cortex in ADHD.
Furthermore, this P3age-effect is consistent, at least in part,withHalperinandSchulz’s(H-S)neurodevelopmental model of ADHD persistence (Halperin and Schulz, 2006), which suggeststhatpartiallydistinct neural andcognitive mechanismsareinvolvedinthecauseofandrecoveryfrom ADHD.
The model assumes that the degree to which the de- velopmentof theprefrontal cortexis abletocompensate for early neural deficits accounts for the improvement of symptoms frequently seen in ADHD with age. We inves- tigated the improvement of a core executive function in ADHD, the BRI, in relation with age, as indexed by the commission-errors. We found that both study groups ex- hibited better performance with increasing age in terms ofcommission-errors,buttheimprovementwasmorepro- nouncedintheADHDsample.Additionally,wefoundapro- longation of RTs with age in both groups, which, in con- trasttothecommission-errors,wasmoresubstantialamong HCs.
WhilepartiallyconsistentwiththeH-Smodel,thefinding thattheP3diminutioneffectwasrestrictedtoacertainage rangepointsbeyondthecompensatoryprocessesconsidered inthemodel.Inparticular,withrespecttotheneurodevel- opmentaltimecourseofP3wefoundadeclineofP3ampli- tudeintheADHDgroup,whichstartedfrommid-adulthood.
Asthefronto-central NoGoP3isconsidered toreflectthe functioningofthefrontalareas(whichshowadelayedmat- urationinADHD),ourfindingsareconsistentwiththe‘‘last in,firstout’’hypothesis (Douaudetal.,2014;Raz, 2005), which refers to a mirroring pattern of development and aging of the human brain. As stated by (Douaud et al., 2014), “thesequence of events associated withbrain de- cline should present itself in reverse order to the series ofeventsrelatedtobraindevelopment,withbrainregions thought to develop relatively late - at both ontogenetic andphylogeneticlevel– alsodegeneratingrelativelyearly"
(Hilletal.,2010; Razetal.,2005;Reisbergetal.,2002).
Hence,ourfindingsraisethepossibilitythatADHD– atleast in patients whose symptoms persist into adulthood - may notonlybeassociatedwithadelayedneuro-developmental trajectory,butalsowithaprematureage-relateddeteriora- tion,atleastinsomeareasofneurophysiologicalfunction- ing.
Although impairment of “hot executive” functions was postulated as a key deficit in ADHD (Barkley, 1997; Surman et al., 2013), previous studies used emotionally- neutralstimuliinNoGoBRItaskstoinvestigatethefronto- centralP3(e.g.,(Fallgatter etal.,2004;Fallgatter etal., 2005;Wiersemaetal.,2006)).Inthecurrentstudy,apply- ingemotionally-neutralaswellasvalencedstimuli,wede- tectedasignificantgroupbyemotionalvalenceinteraction, whichrevealedaneffectofemotionalvalenceintheADHD group:the P3reduction wassignificantlygreater for neg- ativethan for neutralor positive stimuli. Among HCs,we foundnooveralldifferencebyemotionalvalenceacrossall ages.
ThereductionofP3inADHDandthemodulationof the P3 reduction by negative emotional valence may reflect the impairment of hot EFs. These findings can be inter- preted in thecontext of cognitive-energetic model (CEM) (Sergeant, 2005), which postulates that ADHD causes de- fectsacross threelevels of hierarchy, includingattention atlevel1(withcomputationalaspectsandmotoroutput);
energeticpools atlevel 2(effort,arousaland activation);
and,the executive/management system (level 3). We as- sume that the overall reduction of P3 in ADHD is associ- ated with a top level deficit in executive/inhibitorycon- trol(whichwouldbelinkedtoimpairmentsin“cool” EFs).
In light of the evidence that the P3amplitude decreases withincreasedarousal(Polich,2007),wethinkthatthead- ditionalP3reduction inADHD by negativestimulimay be viewedaslinkedtothe alterations in theenergetic pool, includingarousal(atlevel2intheCEM).
According to the low arousal theory of the disorder (Barry etal., 2003a; Barry et al., 2003b), ADHD patients arecharacterizedby hypo-arousal,which hasbeen shown tobeassociatedwitharelativeup-regulationofthephasic arousal-response(SikstromandSoderlund,2007).Thismay lead to hypersensitivity to emotionally-salient environ- mentalstimuli,suchasthenegativepicturesinourstudy.
The behavioral finding that commission-error rates are
(LittmanandTakacs,2017).
Severalissuesandstudylimitationsmeritcomment.First, the response inhibition task we used involves a working memory (WM) component, as it requires a “same” vs.
“different” decision based on the immediately preceding stimulus. It is therefore conceivable that WM alterations presented a confound in our investigation. Further stud- ies thatsystematicallymanipulate theWM demandinthe task are needed to clarify this issue. Second, the possi- bility of premature deterioration in patients with ADHD in certainelectrophysiologicalindicesof functioning,such asthe age-relatedP3reduction,maybe seen atvariance withsomeof theearlierfindings.Forexample,theafore- mentioned mega-analysis of structural-MRI data indicated a later onset of volume decrease in the nucleus accum- bensandputamenintheADHDascomparedtothecontrol group (Hoogmanet al., 2017). However,one has to keep in mindthatvariouscorticaland subcorticalbrainregions have different developmental trajectories (Coupe et al., 2017;Frankeetal.,2018;Storsveetal.,2014).Third,our study included only adults with ADHD, and therefore did not coverthe criticaldevelopmentaltrajectory fromado- lescencetoadulthood.Furthermore,itwascross-sectional, whichraisestheissueofpotentialconfoundingintermsof causalrelationshipsandpossible age-cohorteffects.How- ever, our goalwasnottoidentifycausalassociations,but todescribedevelopmentaltrajectorieswithage.Sincewe continuously sampled an age-rangerather than using dis- tinct age-cohorts, andapplied aregression approach, the age-cohort effects seem less likely.Nonetheless, we con- cur with the conclusions of a recent review of lifespan changesinADHDthat“aseverelackofknowledgeonlifes- panaspectsinADHD stillexists”,andlarge-scaleresearch efforts would be essential to overcome the “knowledge gaps through appropriately granular longitudinal studies”
(Frankeetal.,2018).
Finally,approximatelyhalfofthepatientsreceivedstim- ulantmedication,andaboutone-fourthhadcomorbidities.
However,theinclusionofmedicationandcomorbiditysta- tus inthe analysesdidnotinfluence theprincipalresults.
Finally,sincewefocusedonadultpatients,whosesymptom severity metthediagnostic criteria(i.e.,their ADHDper- sisted),ourresultspertaintothisgroupofpatients.Hence, ourfindingsmaynotgeneralizetopatientswhosesymptoms donotreachthediagnosticthresholdin adulthood.These patientsmayevidenceamorecompleteandearliernarrow- ing of the age-developmentalgap than thosewhoremain
2014;Raz,2005),whichreferstoamirroringpatternofde- velopment and agingof the human brain.Hence, our re- sultsraisethepossibilitythatADHDmaynotonlybeasso- ciatedwithadelayedneuro-developmentaltrajectory,but alsowithaprematureage-relateddeterioration,atleastin someareasofneurophysiologicalfunctioning.
Role of the funding source
Thisstudy wassupportedby theHungarianBrainResearch Program(2017-1.2.1-NKP-2017-0002).The HungarianBrain ResearchProgramhadnofurtherroleinstudydesign;inthe collection,analysisandinterpretationofdata;inthewrit- ingofthereport;andin thedecisiontosubmitthe paper forpublication.
Contributors
AuthorsKakuszi,CzoborandBitterdesignedstudy.Author Kakusziperformedthedatacollectionandpreprocessingof the EEGdata under the supervision of authors Bitterand Czobor.AuthorsKakusziandCzobormanagedtheliterature searchesandanalyses. AuthorSzuromiparticipated inthe clinicaldatacollection.AuthorsCzoborandKakusziunder- took the statistical analysis. Authors Kakuszi and Czobor wrote the first draft of the manuscript. All authors con- tributedtotheinterpretationofthefindings,andhaveap- provedthefinalmanuscript.
Conflict of interest
AuthorsB.Kakuszi,B.Szuromi,I.BitterandP.Czoborde- clarethat theyhave noconflictofinterest withregard to thisstudy.
Acknowledgments
The authors would like to acknowledge the help of the teamoftheOutpatientClinicforAdultswithADHD,atthe SemmelweisUniversity,inBudapest,Hungaryfortheirpar- ticipationintherecruitmentandclinicalexaminationofthe subjects.