F I N E S T R U C T U R A L L O C A L I Z A T I O N O F A D E N I N E N U C L E O S I D E P H O S P H A T A S E A C T I V I T Y I N T H E
S A R C O P L A S M I C R E T I C U L U M O F S T R I A T E D M U S C L E
12
J . R O S T G A A R D A N D Ο . B E H N K E
Institute of Anatomy, University of Copenhagen, and Department of Anatomy, The Royal Dental College, Copenhagen, Denmark
W e wish to report on some recent histochemical experiments we h a v e done on striated muscle. T h e observations emphasize t h e usefulness of cytochemical staining methods for integrating biochemical and physiological information with newer knowledge of cell morphology.
H o w fruitful such an approach can be is obvious. W e h a v e only to think, for example, of t h e wealth of v a l u a b l e information a b o u t lyso- somes provided by the cytochemical studies of Novikoff [31, 32] a n d others, after de D u v e [5] h a d defined lysosomes biochemically. I n the present case the i n t e r p r e t a t i o n of our cytochemical observations on muscle lead us to present a hypothesis a b o u t t h e fine s t r u c t u r a l localization of an intracellular active t r a n s p o r t mechanism, function- ing as a link between an electrical a n d a mechanical process.
Before going into the details of our subject we should like briefly to review some recent significant findings concerning t h e u l t r a s t r u c t u r e of the muscle fiber, a n d the physiology a n d biochemistry of muscle contraction.
T H E U L T R A S T R U C T U R E O F T H E M U S C L E F I B E R
I n striated muscle fibers the cytoplasm (sarcoplasm) fills t h e spaces between t h e contractile myofibrils. T h e cytoplasm contains endoplasmic reticulum or, as it is t r a d i t i o n a l l y called, t h e sarcoplasmic reticulum (SR) of muscle fiber [ 1 , 2, 12, 3 4 ] . T h e SR forms a well- defined continuous system of m e m b r a n e - b o u n d tubules surrounding
1
This work was supported by a grant from the Danish State Research Foundation.
2
T h e following abbreviations are used in this chapter : A M P , A D P , A T P : adenosine-monophosphate, adenosine diphosphate, adenosine triphosphate;
AMPase, ADPase, A T P a s e : adenosinemonophosphatase, adenosinediphosphatase, adenosinetriphosphatase; T P P : thiamine pyrophosphate.
103
104 J . ROSTGAARD AND Ο. B E H N K E
each myofibril like a mesh. The system is m a d e up of units each of which corresponds to a sarcomere of the myofibril. T h e system is interrupted by channels or tubules which traverse t h e muscle fiber perpendicular to its long axis (Fig. 1 ) . I n some muscles this i n t e r r u p - tion t a k e s place a t t h e level of the Ζ lines, in others a t t h e level of the junction of t h e A and I b a n d . T h e transverse t u b u l a r system is called the Τ system [ 1 ] . W h e r e t h e S R meets the Τ system, the SR broadens into two cisterns or terminal sacs, t h u s forming a c h a r a c - teristic t h r e e - p a r t structure termed a t r i a d [ 3 4 ] . I n sections a t r i a d is seen to consist of a central tubule or vesicle with a diameter of
FIG. 1. Schematic summary of observations on histochemically demonstrable nucleoside phosphatases in rat myocardium. For further description see text.
a b o u t 300-400 Â, flanked on either side by two lateral sacs or cisterns of about the same width. The lateral sacs are parts of the SR and are continuous with the SR around the rest of the sarcomere (Fig.
1). Continuity between the Τ system and t h e t e r m i n a l sacs has never been reported. I n fact, there are two m e m b r a n e s a n d a 250-Â space separating the lumens of the two systems. In heart muscle a bipartite structure, a dyad [34], is commonly found. In dyads the tubule of the Τ system is in contact with only one t e r m i n a l sac from t h e sarco- plasmic reticulum (Fig. 1 ) .
I n morphological studies on h e a r t muscle, fixed in osmium t e t r o x - ide, Simpson and Oertelis [ 3 8 ] , and Nelson and Benson [ 3 0 ] , p r e - sented evidence for continuity between the Τ system a n d t h e p l a s m a m e m b r a n e of the muscle cell. F r a n z i n i - A r m s t r o n g [ 1 3 ] , a n d F r a n z i n i - A r m s t r o n g and P o r t e r [14, 15] have t a k e n a d v a n t a g e of t h e improved
P H O S P H A T A S E ACTIVITY I N STRIATED M U S C L E 105 preservation of m e m b r a n o u s structures obtainable by g l u t a r a l d e h y d e fixation [ 3 7 ] . These workers h a v e d e m o n s t r a t e d beyond d o u b t t h a t , in fish skeletal muscle, t h e tubules of the Τ system are invaginations of the p l a s m a m e m b r a n e a n d t h a t t h e lumen of t h e Τ system is t h u s in direct communication with t h e extracellular space. H u x l e y [25]
h a s presented indirect evidence t h a t t h e same is t r u e of frog skeletal muscle, although direct morphological continuity w a s n o t observed.
I n other words, in t h e triadic structure t h e longitudinal SR comes into contact with the i n v a g i n a t e d p l a s m a m e m b r a n e .
W e h a v e observed, in r a t cardiac muscle (Fig. 2 ) ,
3
t h a t t h e longi- t u d i n a l S R surrounding t h e myofibrils a t t h e p e r i p h e r y of t h e fiber also exhibits contact zones with t h e free p l a s m a m e m b r a n e . These contacts are generally observed a t t h e level of t h e Ζ lines, where the plasma m e m b r a n e is deflected into t h e fiber to form t h e Τ system (Figs. 3, 5 ) . W h e r e these contacts are established t h e S R shows sacs or cisterns reminiscent of t h e l a t e r a l elements of t r i a d s , a n d in fact we consider t h e m to be homologous structures. Similar cisterns were observed in relation to t h e intercalated discs, where t h e cell m e m - branes are oriented parallel to t h e myofibrils (Figs. 4 a n d 8 ) . W e have n a m e d t h e cisterns i m m e d i a t e l y b e n e a t h t h e free p l a s m a m e m b r a n e subsarcolemmal cisterns ( S S C ) .
P H Y S I O L O G Y A N D B I O C H E M I S T R Y O F M U S C L E C O N T R A C T I O N
Hill [21, 22] studied t h e contraction of individual myofibrils within a muscle fiber. H e examined t h e hypothesis t h a t this process is initiated by an a c t i v a t i n g substance, which diffuses from t h e surface of t h e fiber to its interior. H i s experiments led to t h e conclusion t h a t the time interval between contraction of superficial a n d of deep m y o - fibrils is insufficient to support such an explanation. Hill concluded t h a t a process, n o t a substance, m u s t conduct t h e signal for contraction inward. T h e experiments by H u x l e y a n d T a y l o r [24] a n d H u x l e y and S t r a u b [23] d e m o n s t r a t e d t h a t local depolarization of t h e p l a s m a m e m b r a n e would only spread into the fiber a n d cause local contraction if the initial local depolarization were a t t h e level of t h e t r i a d . Because the Τ system is the only p a r t of t h e t r i a d t h a t h a s been shown to be in direct continuity with t h e p l a s m a m e m b r a n e , it seems likely t h a t the Τ system provides t h e p a t h w a y for t h e conduction of t h e excitation into t h e fiber.
3
Figures 2-11 are grouped at end of chapter beginning on p. 110.
106 J . ROSTGAARD AND Ο. B E H N K E
T h e r e is now good evidence t h a t electrical depolarization of t h e sarcolemma causes calcium ions to be released from a c o m p a r t m e n t within t h e cell during a contraction-relaxation cycle. T h e calcium ions catalyze the interaction of the actin and myosin filaments by a c t i v a t i n g t h e myosin A T P a s e , a n d contraction t a k e s place. Calcium ions are then removed to a c o m p a r t m e n t somewhere within t h e cell, myofilament interaction t e r m i n a t e s , a n d relaxation occurs [6, 7, 16, 4 2 , 4 3 , 4 4 ] .
H o w calcium ions are released and subsequently removed is not fully understood, b u t the m e m b r a n e s of the triadic structures and the longitudinally oriented sarcoplasmic reticulum seem to be impli- cated in these processes. This point of view is based upon biochemical studies on muscle homogenates. I n several laboratories a so-called sarcotubular fraction has been obtained by differential centrifugation of muscle homogenates. T h e fraction is considered to consist of broken-up sarcoplasmic reticulum and Τ systems. This sarcotubular fraction h a s been shown to possess three specific activities [6, 7, 18, 19, 43] : (1) A strong calcium binding effect; calcium m a y be concen- t r a t e d more t h a n one t h o u s a n d t i m e s ; (2) a relaxing effect on con- tracted myofibrils, originally described by M a r s h [27] ; and (3) adenosinetriphosphatase activity. T h e A T P a s e is t h o u g h t to correspond to the A T P a s e found by Kielley a n d Meyerhof [26] in the " g r a n u l a r fraction" of skeletal muscle homogenates. I t is stimu- lated by magnesium ions and is different from myosin and mitochon- drial A T P a s e [28, 29] ; it is a relatively resistant enzyme [ 2 0 ] .
According to Hasselbach the relaxing effect of t h e granules and vesicles of the s a r c o t u b u l a r fraction on contracted myofibrils is ex- plained by their calcium-binding activity. T h e three activities have been combined in a hypothesis which postulates an A T P - d r i v e n cal- cium p u m p localized to the m e m b r a n e s of t h e sarcotubular fraction
[7, 18, 19, 2 0 ] .
As this theory is based upon biochemical studies on muscle homogenates, very little can be said a b o u t the intracellular localiza- tion of t h e postulated calcium p u m p . I s the p u m p localized to the m e m b r a n e s of the Τ system, or to the m e m b r a n e s of t h e SR, or per- h a p s to some specialized p a r t s of these systems, or are there m e m - branes from other cell components in t h e fraction?
T h e r e is good evidence t h a t calcium p l a y s t h e same role in t h e regulation of contraction a n d relaxation in h e a r t muscle as it does in skeletal muscle [10, 11] a n d t h a t the m e m b r a n e s of t h e s a r c o t u b u -
P H O S P H A T A S E ACTIVITY I N STRIATED MUSCLE 107 lar fraction obtained from h e a r t muscle can a c c u m u l a t e calcium. A t present it is n o t clear whether a recently described calcium p u m p found in cardiac mitochondria also influences t h e calcium concentra- tion in t h e sarcoplasm [ 3 ] .
Because t h e Τ system a p p e a r e d to be t h e t r a n s m i t t e r of excitation into t h e fiber, this tubule h a s been considered to be t h e c o m p a r t m e n t within the cell into which calcium ions were accumulated a n d from which t h e y were released. T h e volume of t h e Τ system, however, is less t h a n 0 . 5 % of t h e volume of the fiber, a n d a p p e a r s to be too small a c o m p a r t m e n t for storing the calcium [ 2 5 ] .
H a s s e l b a c h [20] t r e a t e d frog skeletal muscle briefly with glycerol to destroy t h e surface m e m b r a n e , a n d then incubated t h e muscle in a solution containing A T P , magnesium, oxalate, a n d calcium ions.
An electron-opaque m a t e r i a l p r o b a b l y consisting of calcium oxalate accumulated in t h e t r i a d s , m a i n l y in t h e lateral elements. C o n s t a n t i n et al. [4] obtained similar results in experiments with frog skeletal muscle fibers from which t h e sarcolemma h a d been dissected a w a y . T h e fibers were first exposed to a calcium solution a n d then t r e a t e d with oxalate. Electron microscopy revealed dense deposits, p r o b a b l y precipitations of calcium oxalate, in the t e r m i n a l sacs of t h e SR.
W h e n t h e oxalate t r e a t m e n t was omitted no deposits were found.
These studies indicate t h a t t h e t e r m i n a l sacs are differentiated p a r t s of t h e SR, with a capacity for a c c u m u l a t i n g calcium, a n d are t h u s p r e s u m a b l y the specific region for t h e site of the calcium p u m p .
Another a p p r o a c h to t h e problem of the intracellular localization of the calcium p u m p would be to d e m o n s t r a t e t h e fine structural localization of t h e A T P a s e energizing this p u m p .
A L D E H Y D E - R E S I S T A N T A T P A S E I N S A R C O P L A S M I C R E T I C U L U M
As mentioned previously, t h e s a r c o t u b u l a r fraction obtained b y differential centrifugation of muscle homogenates possesses A T P a s e activity. Our studies on striated muscle were designed to localize this enzymatic a c t i v i t y in the sarcoplasmic reticulum more precisely b y histochemical m e a n s .
Our experiments were done on r a t s a n d mostly on cardiac muscle.
T h e h e a r t was fixed in situ b y short-time perfusion of t h e coronary arteries with g l u t a r a l d e h y d e . I n the studies on skeletal muscle t h e whole animal w a s fixed b y perfusion with g l u t a r a l d e h y d e or h y d r o x y - adipaldehyde [37] via t h e ascending aorta, whereafter samples of intercostal or psoas muscle were removed. Frozen sections from t h e
1 0 8 J . ROSTGAARD AND Ο. B E H N K E
fixed h e a r t and skeletal muscle were then incubated for 15-45 m i n u t e s a t room t e m p e r a t u r e a n d p H 7.2, in a W a c h s t e i n - M e i s e l - t y p e m e d i u m [41] containing A T P , A D P , A M P , or T P P (Sigma) as s u b s t r a t e , and lead ions as c a p t u r e reagent. After incubation the tissues were postfixed in osmium tetroxide and embedded in E p o n . T h i n sections were examined in a Siemens E l m i s k o p I, either unstained or stained with lead citrate [ 3 5 ] .
A more detailed description of the technique used has been p r e - sented elsewhere [ 3 6 ] .
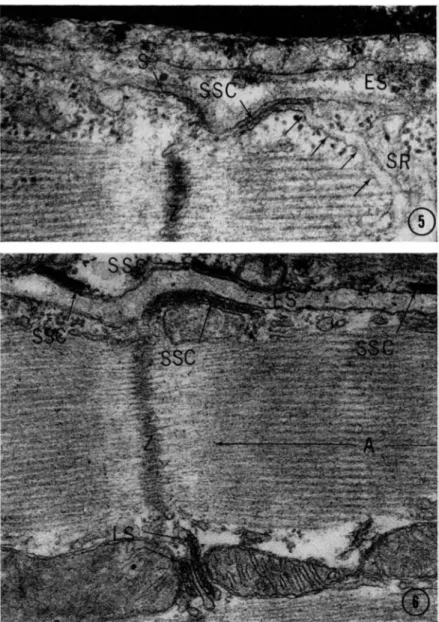
Figure 2 shows an electron micrograph of h e a r t muscle incubated with A T P as substrate. Electron-dense deposits of reaction product are seen in the lateral sacs of the triadic structures. T h e Τ system is entirely free of reaction product. Figures 3 and 6 d e m o n s t r a t e t h a t the subsarcolemmal cisterns were also reactive when A T P was used as substrate, both at the free surface of the fiber (Fig. 3) and a t the intercalated discs (Fig. 4 ) . This supports the assumption t h a t the subsarcolemmal cisterns are homologous with the t e r m i n a l cisterns.
T h e A T P a s e in the t e r m i n a l sacs and the subsarcolemmal cisterns was relatively resistant to aldehyde fixation. J u d g e d by the a m o u n t of reaction product, more enzymatic activity was retained when the h e a r t muscle was fixed by perfusion for a shorter period of time, with lower concentrations of the fixative. T h e reaction product, how- ever, was limited to the same fine structural elements. M y o s i n and mitochondrial A T P a s e could n o t be demonstrated in the g l u t a r a l d e - hyde-fixed m a t e r i a l . I t has been shown by others [40] t h a t these A T P a s e s are very sensitive to fixatives. This is in agreement with the biochemical observation [20] t h a t s a r c o t u b u l a r A T P a s e (so-called basic A T P a s e ) is more résistent to inhibitors than myosin ATPase.
When aldehyde-fixed skeletal muscle was incubated for demonstration of A T P a s e activity, the reaction product was also found in the lateral elements of triads. Figure 11 is a longitudinal section of rat psoas muscle, fixed by perfusion with hydroxyadipaldehyde. The preserva- tion of fine structure is far inferior to t h a t obtainable with glutaralde- hyde (Fig. 10). The myofibrils appear contracted, but it is evident that reaction product is present in the lateral elements of the triads, which in this muscle are found at the A-I junction. For comparison
a section of unincubated psoas muscle is shown in Figure 10.
With A D P as substrate, deposits of reaction product were observed in the same structural elements as with A T P but in far lesser amount.
When sections were incubated in AMP-containing media no final
P H O S P H A T A S E ACTIVITY I N STRIATED MUSCLE 109 product was observed in lateral elements of t r i a d s or in the subsar- colemmal cisterns. However, deposits of final p r o d u c t were consis- tently observed in the i n t e r m e d i a t e elements of t r i a d s (Fig. 7) a n d in the interspace between t h e p l a s m a m e m b r a n e s of t h e i n t e r c a l a t e d discs (Fig. 8 ) . T h e " t i g h t " junctions a t t h e intercalated discs showed no e n z y m a t i c a c t i v i t y .
W i t h T P P as s u b s t r a t e no reaction p r o d u c t was found in a n y structures in h e a r t muscle. Likewise, control sections incubated in lead-containing media b u t without s u b s t r a t e showed no lead adsorp- tion to cellular structures (Fig. 9 ) .
C O N C L U D I N G R E M A R K S
I n all our experiments t h e incubation media were filtered j u s t before use and no spontaneous precipitation occurred. Therefore, the deposits of lead p h o s p h a t e inside t h e t e r m i n a l sacs and subsarcolem- mal cisterns m u s t be t h e result of the a c t i v i t y of an enzyme capable of splitting A T P . T h e w
r
eak reaction observed with A D P as s u b s t r a t e m a y be due to impurities of A T P . T h e s a r c o t u b u l a r A T P a s e demon- s t r a t e d by light microscopy [8, 33] in unfixed h u m a n skeletal muscle fibers p r o b a b l y corresponds to the s a r c o t u b u l a r p h o s p h a t a s e found in our experiments. C e r t a i n l y the similarity of t h e distribution of A T P a s e , in a fine reticular p a t t e r n a t t h e level of t h e triadic s t r u c - tures, is suggestive. F u r t h e r m o r e , the v e r y reactive regions adjacent to the sarcolemma a t the level of the t r i a d s [8] m a y correspond to the reactive subsarcolemmal cisterns we have observed. Consistent with our results, Engel [8] also observed a slight reaction with A D P as s u b s t r a t e .
Until recently only a few electron microscopic studies of p h o s p h a - tase activity in muscle h a v e been published. Sommer a n d Spach [39]
studied nucleoside phosphatases in cardiac a n d skeletal muscle of the dog, and found t h a t t h e reaction p r o d u c t a p p e a r e d to be localized in the space between the t e r m i n a l sacs and the t u b u l e of the Τ system.
Similar localization of final product was n o t noted in our p r e p a r a t i o n s of r a t m y o c a r d i u m . W e a l w a y s found A T P a s e a c t i v i t y within t h e terminal sacs, and this is consistent with a v e r y recent r e p o r t of Essner, Novikoff, a n d Q u i n t a n a [ 9 ] . T h e experiments b y these a u t h o r s were also carried out on r a t m y o c a r d i u m and their results with A T P were essentially similar to those described in the present report.
U n d e r the experimental conditions employed, our cytochemical studies suggest the following conclusions: (a) T h e sarcoplasmic retic-
110 J . ROSTGAARD AND Ο. B E H N K E
FIG. 2. Frozen section of r a t myocardium fixed by perfusion with glutaralde- hyde and incubated for 45 minutes in a medium containing A T P , lead ions, and magnesium ions. Electron-dense deposits of reaction product are seen in the lateral sacs (LS) of sarcoplasmic reticulum of dyads and triads located at the level of the Ζ bands (Z). T h e Τ system (TS) and longitudinally oriented sarcoplasmic reticulum (SR) are nonreactive. A, A band. SSC, subsarcolemmal cistern. Magnification: X 26,000. T h e insert shows a higher magnification of a triad from the same experiment. Only the lateral sacs (arrows) show ATPase activity, M , mitochondria. Magnification: X 52,000.
FIG. 3 . R a t myocardium incubated for 4 5 minutes with A T P as substrate.
The micrograph demonstrates continuity between the lumen of the Τ system (TS) and the extracellular space ( E S ) . Four subsarcolemmal cisterns (SSC) show ATPase activity. Reaction product is also seen in lateral sacs (LS) of dyads and trials. Ζ, Ζ bands. Magnification : X 33,000.
FIG. 4 . Myocardial tissue incubated for the demonstration of ATPase ac- tivity. T h e micrograph shows an intercalated disc (a,b,c,d). Where the plasma membranes of the cells are oriented parallel to the myofibrils, and form longitudi- nal connecting surfaces (b,c), reactive subsarcolemmal cisterns (SSC) are found in intimate contact with the plasma membranes. N o activity is seen in the narrow interspace between the adjacent cells (compare with Fig. 8 ) . T, reactive triad. Magnification: X 33,000. (From [ 3 6 ] courtesy of J. Ultrastruct. Res.)
FIG. 5. Unincubated rat myocardium fixed by perfusion with glutaraldehyde and postfixed in osmium tetroxide. T h e micrograph shows a subsarcolemmal cistern (SSC) located immediately beneath the sarcolemma (S). T h e subsar- colemmal cistern exhibits direct continuity (arrows) with the longitudinally oriented sarcoplasmic reticulum ( S R ) . Ζ, Ζ band. E S , extracellular space. N , nucleus of an endothelial cell. Magnification : X 70,000.
FIG. 6. Tissue incubated as described in legend to Fig. 2. T h e extracellular space (ES) between two muscle fibers is seen. Four subsarcolemmal cisterns (SSC) located immediately beneath the plasma membrane show ATPase activity.
N o t e reactive lateral sacs (LS), Ζ and A bands. Magnification: χ 52,000.
PHOSPHATASE ACTIVITY I N STRIATED MUSCLE 113
FIG. 7. Myocardium incubated 45 minutes in a medium containing A M P , lead ions and magnesium ions. Reaction product is seen in the lumen of t h e Τ system (TS) of triads but not in the lateral sacs. T h e extracellular space (ES) and a few vesicles (V) in the peripheral sarcoplasm show reaction product.
N , nucleus of endothelial cell. Ζ, Ζ band. A, A band. Magnification: χ 43,500.
114 J . ROSTGAARD AND Ο. B E H N K E
FIG. 8. Myocardium incubated with A M P as described for Fig. 7. T h e micro- graph shows an intercalated disc ( I D ) . Reaction product is seen in the extracellu- lar space (ES) and in the narrow interspace (IS) between the cell membranes of the adjacent cells, but not at the "tight" junction ( T J ) . N o t e nonreactive subsarcolemmal cistern (SSC). A and Ζ bands are marked. Magnification:
X 43,500.
FIG. 9. Control section of myocardium incubated for 45 minutes in a medium containing lead and magnesium ions, b u t no substrate. N o lead deposits are seen in triads ( T ) , myofibrils, or mitochondria. Magnification: X 33,000.
P H O S P H A T A S E A C T I V I T Y I N S T R I A T E D M U S C L E 115
FIG. 10. Nonincubated skeletal muscle from rat, fixed in glutaraldehyde, postfixed in osmium tetroxide, and stained with lead citrate. Typical triads (arrows) can be seen at the level of the A-I junction. Ζ, Ζ band. A, A band.
Magnification: X 43,500.
FIG. 11. Frozen section of skeletal muscle from rat, fixed by perfusion with hydroxyadipaldehyde and incubated in a medium containing A T P , lead ions, and magnesium ions. Reaction product is seen in the lateral elements of triadic structures (arrows). T h e preservation of the intracellular membranes is inade- quate with this fixative. For interpretation compare with Fig. 10. Ζ, Ζ band.
A, A band. Magnification: X 52,000.
P H O S P H A T A S E ACTIVITY I N STRIATED MUSCLE 117 u l u m is not a uniform system, inasmuch as some p a r t s of t h e SR, i.e., the t e r m i n a l sacs a n d t h e subsarcolemmal cisterns, differ enzy- m a t i c a l l y from t h e rest of t h e SR. (6) I t is possible to discriminate between the different components of t r i a d s and d y a d s ; A T P a s e is found in the t e r m i n a l sacs a n d an enzyme splitting A M P in t h e Τ system, (c) T h e flat cisterns ( S S C ) , located immediately beneath the sarcolemma a t the Z-line level, a p p e a r to be e n z y m a t i c a l l y and morphologically homologous to the lateral sacs of the t r i a d s .
Our experiments also d e m o n s t r a t e t h a t the s a r c o t u b u l a r fraction m a y be far less homogeneous t h a n is usually t h o u g h t to be the case, and t h a t t h e activities a t t r i b u t e d to this fraction m a y be restricted to the terminal sacs a n d SSC's.
We are well a w a r e of t h e fact t h a t we h a v e n o t proved t h a t the A T P a s e d e m o n s t r a t e d cytochemically in our experiments is identical with the A T P a s e of the s a r c o t u b u l a r fraction related to the calcium p u m p . T o settle this problem more experimental work using enzyme inhibitors and a c t i v a t o r s has to be done, b u t u n f o r t u n a t e l y q u a n t i t a - tive cytochemical studies at the electron microscopic level are difficult to perform a n d interpret. M o s t inhibitors used cause only a p a r t i a l inhibition of e n z y m a t i c a c t i v i t y a n d this is difficult to recognize in the electron microscope. T h e r e is much u n c e r t a i n t y regarding t h e n a - t u r e of the A T P a s e studied. Nevertheless, the i n t i m a t e relationship of the reactive structures to the free surface of the cell, to t h e Τ system, a n d to t h e i n t e r c a l a t e d disc signify t h a t t h e y function in the coupling of excitation a n d contraction, and, furthermore, t h a t the A T P a s e - r e a c t i v e lateral sacs and the subsarcolemmal cisterns are t h e sites where t h e calcium p u m p is localized.
REFERENCES
1. Andersson-Cedergren, E., J. Ultrastruct. Res. (Suppl. 1) (1959).
2. Bennett, H . S., in " T h e Structure and Function of Muscle" (G. H . Bourne, ed.), Vol. I, p. 137. Academic Press, New York, 1960.
3. Brierly, G. P., in "Energy-linked Function of Mitochondria" (B. Chance, ed.), p. 237. Academic Press, New York, 1963.
4. Constantin, L. L., Franzini-Armstrong, C , and Podolsky, R. J., Science 147, 158 (1965).
5. de Duve, C , in "Subcellular Particles" ( T . Hayashi, ed.), p. 128. Ronald Press, New York, 1959.
6. Ebashi, S., Progr. Theoret. Phys. (Kyoto) (Suppl. 17), 35 (1961).
7. Ebashi, S., and Lipmann, F., J. Cell Biol. 14, 389 (1962).
8. Engel, W. K , Nature 2 0 0 , 588 (1963).
9. Essner, E., Novikoff, A. B., and Quintana, N . , «/. Cell Biol 2 5 , 201 (1965).
118 J . ROSTGAARD AND Ο. B E H N K E 10. Fanburg, B., Federation Proc. 2 3 , 922 (1964).
11. Fanburg, B., Finkel, R. M., and Martonosi, Α., J. Biol. Chem. 239, 2298 (1964).
12. Fawcett, D . W., and Revel, J. P., J. Biophys. Biochem. Cytol. 1 0 (Suppl. 4), 89 (1961).
13. Franzini-Armstrong, C , Federation Proc. 2 3 , 887 (1964).
14. Franzini-Armstrong, C , and Porter, K. R., J. Cell Biol. 2 2 , 675 (1964).
15. Franzini-Armstrong, C , and Porter, K. R., Nature 2 0 2 , 355 (1964).
16. Hasselbach, W., Naturwissenschaften 5 0 , 249 (1963).
17. Hasselbach, W., in "Progress in Biophysics and Molecular Biology" (J. A.
V. Butler and Η . E . Huxley, eds.), Vol. 14, p. 169. Macmillan (Pergamon), New York, 1964.
18. Hasselbach, W., and Makinose, M., Biochem. Z. 333, 518 (1961).
19. Hasselbach, W., and Makinose, M., Biochem. Z. 339, 94 (1963).
20. Hasselbach, W., Federation Proc. 23, 909 (1964).
21. Hill, Α. V., Proc. Roy.Soc (London) Ser. B135, 446 (1948).
22. Hill, Α. V., Proc. Roy. Soc. (London) Ser. B136, 399 (1949).
23. Huxley, A. F., and Straub, R. W., J. Physiol. (London) 143, 40 (1958).
24. Huxley, A. F., and Taylor, R. E., J. Physiol. (London) 1 4 4 , 426 (1958).
25. Huxley, Η. E., Nature 202, 1067 (1964).
26. Kielley, W. W., and Meyerhof, O., J. Biol. Chem. 176, 591 (1948).
27. Marsh, Β. B., Nature 167, 1065 (1951).
28. Muscatello, U., Andersson-Cedergren, E., Azzone, G. F., and von der Decken, A , J. Biophys. Biochem. Cytol. 1 0 , (Suppl. 4), 201 (1961).
29. Nagai, T., Makinose, M., and Hasselbach, W., Biochim. Biophys. Acta 4 3 , 223 (1960).
30. Nelson, D . Α., and Benson, E . S., J. Cell Biol. 16, 297 (1963).
31. Novikoff, A. B., in " T h e Cell" (J. Brachet and A. E . Mirsky, eds.) Vol.
I I , p. 423. Academic Press, New York, 1961.
32. Novikoff, A. B., in "Ciba Foundation Symposium on Lysosomes" (Α. V.
S. de Reuck and M. P . Cameron, eds.), p. 36. Churchill, London, 1963.
33. Padykula, H. A , and Gauthier, G. F , J. Cell Biol. 1 8 , 87 (1963).
34. Porter, K. R., and Palade, G. E., J. Biophys. Biochem. Cytol. 3, 269 (1957).
35. Reynolds, E. S., J. Cell Biol. 17, 208 (1963).
36. Rostgaard, J., and Behnke, O., J. Ultrastruct. Res. 1 2 , 579 (1965).
37. Sabatini, D . D., Bensch, K. G., and Barrnett, R. J., / . Cell Biol. 1 7 , 19 (1963).
3 3 . Simpson, F . O., and Oertelis, S. J., J. Cell Biol. 1 2 , 91 (1962).
39. Sommer, J. R., and Spach, M . S., Am. J. Pathol. 4 4 , 491 (1964).
40. Tice, L. W., and Barrnett, R. J., J. Cell Biol. 1 5 , 401 (1962).
41. Wachstein, M., and Meisel, E., Am. J. Clin. Pathol. 27, 13 (1957).
42. Weber, Α., J. Biol. Chem. 234, 2764 (1959).
43. Weber, Α., and Herz, R., in "Biochemistry of Muscle Contraction" (J.
Gergely, ed.), p. 222. Little, Brown, Boston, Massachusetts, 1964.
44. Weber, Α., and Foothills, J. C , J. Biol. Chem. 235, 2500 (1960).