651
The Importance of the Measurement of Enzyme Activity in Medicine
E. Schmidt, F. W. Schmidt, H. D. Horn and U. Gerlach
A) Principles
1. Classification of enzymes in blood plasma 2. Release of enzymes from cells
3. Enzyme patterns and their changes in disease 4. Isoenzymes and their properties
B) Diagnosis, following the course of diseases, control of therapy, and prognosis I. Enzyme activity in serum
1. Diseases of the liver a) Acute hepatitis
b) Chronic hepatitis and cirrhosis of the liver c) Hepatitis due to other causes
d) Toxic liver damage e) Liver congestion f) Obstructive jaundice
g) Primary and secondary liver tumours
h) Assay o f enzyme activity in serum during the prophylaxis and therapy of liver diseases
i) Routine tests in diseases of the liver 2. Diseases o f the heart
a) Myocardial infarction
b) Effort syndrome, angina pectoris, coronary insufficiency c) Pericarditis, myocarditis, myocardial insufficiency
d) Differential diagnosis between pulmonary infarction and pancreatitis e) The evaluation of the assay o f enzyme activity in serum in heart diseases 3. Diseases of striated muscle
a) Primary diseases of muscle b) Secondary diseases of muscle
c) Enzyme activity in serum and the clinical picture in muscle disease d) Pathophysiological information
e) The evaluation of the assay of enzyme activity in serum in muscle diseases 4. Diseases of blood
a) Anaemias
b) Polycythaemia and polycythaemia vera c) Leukaemia
5. N o r m a l values for enzyme activity in human serum
652 Section C : Measurement of Enzyme Activity
II. Enzyme activity in cerebrospinal fluid 1. Cerebro-vascular diseases
2. Primary or metastatic tumours of the central nervous system 3. Meningitis and meningo-encephalitis
4. Other diseases of the central nervous system
5. N o r m a l values for enzyme activity in cerebrospinal fluid III. Enzyme activity in urine
IV. N o t e s on the technique of enzyme assays
A) Principles
The determination of enzyme activity has been an established part of clinical diagnosis for several decades. Since the observation of Warburg (1943)
1
* that enzymes involved in tissue metabolism also occur in serum, the number of these assays has greatly increased, particularly recently.
1. Classification of enzymes in blood plasma
To classify the numerous enzymes which have been detected in blood plasma Biicher
2
,^
proposed that for biological purposes the enzymes should be arranged according to their function and source and not according to their enzyme action (see Table 1).
Table 1. Classification of the enzymes present in blood plasma according to function and source Group
Plasma-specific enzymes Secreted enzymes
Cellular enzymes (enzymes of tissue metabolism) Enzymes of key metabolic pathways
Organ-specific enzymes
Examples
prothrombin, plasminogen, caeruloplasmin, lipoprotein lipase, pseudocholinesterase pancreatic, parotid-a-amylase; prostatic phos
phatase, pepsinogen ,
lactic, malic and a-glycerophosphate dehydro
genase; 1,6-diphosphofructoaldolase; glutamate- oxaloacetate and glutamate-pyruvate transami
nase
Liver: urea cycle enzymes, sorbitol dehydro
genase, 1-phosphofructoaldolase, glucose- 6-phosphatase
B o n e : alkaline phosphatase
The "plasma-specific enzymes" are secreted in an active form into the plasma which is their site of action. The main examples are the enzymes involved in blood coagulation. Their activity in plasma decreases when the organs from which they originate are damaged.
The catalytic activity of the "secreted enzymes" and "cellular enzymes" is not necessary for the function of plasma. These enzymes are only found in plasma because the blood circulates through areas of high enzyme activity. In certain pathological conditions the "se
creted enzymes" can be clearly associated with their source. Their determination inaugurated
1) O. Warburg and W. Christian, Biochem. Z. 314, 399 [1943].
2) Th. Biicher and P. Baum, Lecture, Dtsch. Kongr. f. arztl. Fortbildung, Berlin 1958.
3) Th. Biicher, E. Schmidt and F. W. Schmidt, Lecture, 9th Middle East Assembly, Beirut 1959.
L a In Medicine 653
the use of enzymes in clinical diagnosis. The group of "cellular enzymes" includes the enzy
mes of tissue metabolism, which are not active in plasma or serum because their coenzymes and most of their substrates are absent. The majority of the enzymes in this group, which have been studied from a clinical standpoint, belong to the main energy-yielding metabolic pathways, that is they are present in all tissues of the organism. Certain enzymes of cell metabolism are mainly associated with a particular organ so that inferences can be drawn about the state of the organ from the occurrence of these enzymes in serum. These enzymes are the so-called "organ-specific enzymes".
The greatest advance in the use of enzymes as aids to diagnosis has originated from the study of enzymes involved in tissue metabolism. Therefore this survey deals mainly with the results of pathophysiological and clinical studies on these enzymes.
2. Release of enzymes from cells
It is usually assumed that the reason for the increase of enzyme activity in serum is due to damage to the cells of an organ. Studies on experimental animals have shown that there is a far-reaching correlation between the increase in activity of individual enzymes, particu
larly transaminases, in serum and the dose of a toxin
4 - 7
\ the concentration and virulence of an injected virus
8)
or the size of the area of supply of a ligatured vessel9-n). Clinical studies have also shown agreement between the severity of the disease and the extent of the enzyme changes in s e r u m
1 2 _ 1 4 )
. The release of enzymes from cells was established by findings on isolated o r g a n s
1 5 _ 2 0 )
, by the agreement between the characteristic properties of the enzymes occurring in serum with those of the enzymes of the damaged o r g a n
2 1 - 2 4
) and by the decrease in enzyme activity in the damaged organ2 5 _ 2
9 ) .
F. Wroblewski and / . S. LaDue, Cancer 8, 1155 [1955].
D. W. Molander, F. Wroblewski and / . S. LaDue, J. Lab. clin. Med. 46, 831 [1955].
F. H. Bruns and / . Neuhaus, Biochem. Z. 326, 242 [1955].
M. Asada, Med. J. Osaka Univ. 9, 45 [1958].
C. Friend, F. Wroblewski and J. S. La Due, J. exp. Medicine 102, 699 [1955].
/. Nydick, F. Wroblewski and / . S. LaDue, Circulation 12, 161 [1955].
/. Nydick, P. Ruegsegger, F. Wroblewski and J. S. LaDue, Circulation 15, 324 [1957].
L. A. Rudolph, J. A. Schaejfer, R. E. Dutton and R. H. Lyons, J. Lab. clin. Med. 49, 31 [1957].
F. DeRitis, A. Ascione, M. Coltorti, G. Giusti and L. Malluci, Giorn. malatt. infett. parasitt. 11, 1 [1959].
F. Wroblewski, Amer. J. Med. 27, 911 [1959].
/. Beyreder and H. Rettenbacher-Daubner, Wien. Klin. Wschr. 686 [1959].
K. L. Zierler, Amer. J. Physiol. 185, 12 [1956].
K. L. Zierler, Amer. J. Physiol. 190, 201 [1957].
K. L. Zierler, Amer. J. Physiol. 192, 283 [1958].
K.L. Zierler, Bull. Johns Hopkins Hosp. 102, 17 [1958].
K. L. Zierler, A n n . N . Y. Acad. Sci. 75, 227 [1958].
/. A. Sibley, A n n . N . Y. Acad. Sci. 75, 339 [1958].
Th. Wieland, G. Pfleiderer, I. Haupt and W. Worner, Biochem. Z. 332, 1 [1959].
R. J. Wieme: Studies on Agar Gel Electrophoresis. Arscia Uitgaven, Brussels 1959.
B. Hess: Verhandlungen, I. Europaisches Symposium Med. Enzymologie, Milan 1960. S. Kar- ger, Basle 1961, p. 83.
E. S. Vesell and A. G. Beam, J. clin. Invest. 40, 586 [1961].
J. C. Dreyfufi, G. Shapira, F. Shapira and / . Demos, Clin. chim. Acta / , 434 [1956].
R. B. Jennings, J. P. Kaltenbach and G. W. Smetters, Arch. Pathology 64, 10 [1957].
F. Wroblewski, Ann. N . Y. Acad. Sci. 75, 322 [1958].
E. Schmidt, F. W. Schmidt and E. Wildhirt, Klin. Wschr. 36, 280 [1958].
P. E. Strandjord, K. E. Thomas and L. P. White, J. clin. Invest. 38, 2111 [1959].
654 Section C: Measurement of Enzyme Activity
Different views have been put forward as to the extent of the cellular changes which lead to the liberation of enzymes from the cells. According to the simplest hypothesis, on the death of the cell the enzymes pass down the concentration gradient into the extracellular space and the plasma. The correlation between the degree of necrosis
3 0 ,
3 1
) or the size of the area of infarction
1 0)
and the extent of increase in enzyme activity in the serum is put forward as proof of this conception. However, this correlation is by no means constant
2 0 .
3 2
), and the amount of visible necrosis is only an indication of the general cell damage to the organ or part of the organ. Numerous findings indicate that even very slight cell damage, which is not detectable morphologically, is accompanied by the release of enzymes from the cells. For example, after hypoxia, there is a considerable rise in the enzyme activity in serum after a short time
2 8 ,
3 4
), although the structural changes are only visible after 4 —6 hours 3 3 )
. It is even questionable whether the natural death of the cell during normal cell turnover is always coupled with the liberation of enzymes. As a rule no changes in the enzyme levels in serum are observed in either tissue break-down during starvation
3 5
) or in extensive neuro
muscular atrophy 3 6 -
"
3 8
). By contrast, in severe damage to the cell metabolism, as in myositis or congenital muscular dystrophy, the changes in the enzyme levels form part of the picture of the disease
3 6
*
3 8 - 4 1
) (see also "Diseases of striated muscle", p. 688).
" Considering the very great differences in enzyme concentrations between the intra and extra
cellular space, and the extraordinarily large surface of the cells compared with the space occupied by the plasma, it is an astonishing phenomenon that the plasma is so poor in enzymes" (Biicher V). This shows the efficiency of the cell membrane. Its existence and func
tion are inseparable from the energy metabolism of the cell. Release of enzymes is therefore to be expected when the energy available to the cell is no longer sufficient for its needs. This is shown particularly clearly by comparison of the release of enzymes with the mitochondrial damage in rats poisoned with carbon tetrachloride (Fig.l). The marked increase of enzyme activity in serum coincides with the greatest amount of mitochondrial damage
4 2 ,
4 3 ). Nume
rous studies on ascites tumour c e l l s 1
,
4 4 - 4 8
) , on isolated organs and on experimental animals have shown that restriction of metabolic activity (e.g. by anaerobiosis or CO poisoning,
30) / . M. Merill, J. Lemley-Stone, J. T. Grace and G. R. Meneely, Amer. J. Med. 160, 1454 [1956].
3D S. Zelman, Ch. Wang and / . Appelhanz, Amer. J. med. Sci. 237, 323 [1959].
32) R. L. Sterkel, J. A. Spencer, S. K. Wolfson and H. G. Williams-Ashman, J. Lab. clin. Med. 52, 176 [1958].
33) G. Holle, R. Burckhardt, S. Arndt and M. Bloedorn, Virchows Arch, pathol. A n a t o m . Physiol.
klin. Med. 327, 150 [1955].
34) B. Hess and R. Raftopoulo, Dtsch. Arch. klin. Med. 204, 97 [1957].
35) L. P. White, Ann. N . Y. Acad. Sci. 75, 349 [1958].
36) W. Jakob and / . Neuhaus, Klin. Wschr. 32, 923 [1954].
37) C. M. Pearson, N e w England J. Med. 256, 1069 [1957].
38) / . C. Dreyfufi, G. Shapiro and F. Shapiro, A n n . N . Y. Acad. Sci. 75, 235 [1958].
39) / . C. Dreyfufi and G. Shapiro, J. clin. Invest. 33, 794 [1954].
40) / . C. Dreyfufi and G. Shapiro, C. R. Seances Soc. Biol. Filiales 147, 1145 [1953]; 149, 1934 [1955].
41) R. G. Siekert and G. A. Fleisher, Proc. Staff Meetings M a y o Clin. 31, 459 [1956].
42) D. N. Calvert and T. M. Brody, J. Pharmacol, exp. Therapeut. 124, 213 [1958].
43) R. O. Recknagel and D. D. Anthony, J. biol. Chemistry 234, 1052 [1959].
44) O. Warburg and E. Hiepler, Z. Naturforsch. 7b, 193 [1952].
45) A.L. Schade, Biochim. biophysica Acta 12, 163 [1953].
46) O. Warburg, K. Gawehn and G. Lange, Z. Naturforsch. 9b, 109 [1954].
47) 7?. Wu and E. Racker, Fed. Proc. 17, 399 [1958].
48) R. Wu, Cancer Res. 19, 1217 [1959].
In Medicine 655
substrate deficiency or metabolic inhibitors, such as iodoacetate, fluoroacetate, dinitrophenol and salyrgan) generally leads to release of enzymes from c e l l s
1 5 - 2 0
,4 9 - 5 1 ) . According to the type of damage, differences in the extent of the release and in the rate of diffusion of the enzymes can be observed
4 8
,5 1
).Not only injury, but also increase of specific cellular capacity acts as a stimulus for the release of enzymes. For example, Zierler
19
** found that about 10% of the aldolase was released after
Hours
Hours
Fig. 1. (a) Rise in G O T activity in serum and (b) mitochondrial damage in rats poisoned with CCl4
4 2 > 4 3 )
(a) Peak of the CCU concentration in the liver at A
(b) Left-hand ordinate (open panels): Quotient D N P stimulated A T P a s e / M g
2+
stimulatedATPase Right-hand ordinate (shaded panels): Increase [%] in the mitochondrial 02 uptake after addition
of 2,4-dinitrophenol ( D N P )
tetanic excitation of a muscle in situ. The suggested explanation is that a change in the proper
ties of the cell membrane during or after the muscle contraction allows more rapid admission of substrates and a more rapid exit of the end-products, while also permitting the release of cellular enzymes. This conception could also explain the increase in enzyme activity in serum after muscular exercise
5 2
,5 3 )
or the occasional reports of changes in the enzyme levels after large meals
5 4
>
5 5 )
4 .9) F. H. Brum, Clin. chim. Acta 2, 257 [1957].
50) F. H. Bruns and / . Neuhaus, Arch. Biochem. Biophysics 55, 588 [1955].
51
) F. H. Bruns, E. Brosswitz, H. Dennemann, H. D. Horn and E. Noltmann, Klin. Wschr. 39, 342 [1961].
52
) E. Schmidt and F. W. Schmidt in E. Wddhirt: Fortschritte der Gastroenterologie. Urban und Schwarzenberg, Munich 1960.
53) H. Loll and A. Hdscher, Arztl. Forsch. 12, II 85 [1958].
54
) R. Richterich: Enzymopathologie. Springer, Heidelberg 1958.
55) A. Engelhardt-Golkel, R. Lobel, W. Seitz and /. Woller, Klin. Wschr. 36, 462 [1958].
656 Section C : Measurement of Enzyme Activity
The release of enzymes when cell metabolism changes because of disease is therefore only one reason for the increase of enzyme activity in serum. However, in medical practice changes brought about by disease are most interesting and from the quantitative aspect these changes are most important. How far in the cases mentioned the same mechanisms lead to a release of enzymes is not yet clear. Similarly the question, whether the increase in metabolism ob
served at the start of cell damage causes a release of enzymes comparable to that occurring on muscular exercise or whether finally with advancement of the damage the structure of the cell can no longer be maintained, remains to be clarified.
3. Enzyme patterns and their changes in disease
Different organs differ quantitatively and also in part qualitatively in their enzyme make up (see Table 2a and 2b, p. 658, 659).
MDH
ADH SDH (a)
MDH
JSIDHADHSDH y
(b) 100
10 h
GPT
/ /
GOT^^
MDH
\\\
SDH\\ADH
\GIDH GOT
^^rGlDH
\SDH GPT ]ADH PK
PK
10
s
10"
10
J
fc) (d) 10
2
Fig. 2. Enzyme activity (a) in normal heart muscle, (b) in serum in myocardial infarction, (c) in serum in hepatitis, (d) in normal l i v e r
5 7
) . Outer ordinate: Enzyme activity in Biicher units/100 mg. soluble protein Inner ordinate: Enzyme activity in Biicher units/ml. serum
For the meaning o f the abbreviations, see under Table 2 (p. 658, 659)
These "enzyme patterns" are characteristic for a particular organ and allow/identification without recourse to morphological methods
5 6
). The pattern of the enzyme
n
found in serum if the parenchymal cells of a particular organ are injured by disease is characteristic and clearly different from that which occurs if another organ is diseased2.3,52,57-59) (Fig. 2). The simi-
56) J. P. Greenstein: Biochemistry of Cancer. 2nd. ed., Academic Press, N e w York 1954.
5
7) E. Schmidt and F. W. Schmidt: Verhandlungen I. Europaisches Symposium Med. Enzymologie, Milan 1960. S. Kargei, Basle 1961, p. 100.
58) E. Schmidt and F. W. Schmidt, Lecture, Kongr. Dtsch. Gastroenterolog. Ges., Zurich 1960.
Bibl. gastroenterol. S. Karger, Basle 1961, vol. 4, p. 15.
59) H. Kalk, E. Schmidt, F. W. Schmidt and E. Wildhirt, Klin. Wschr. 38, 421 [I960].
In Medicine 657
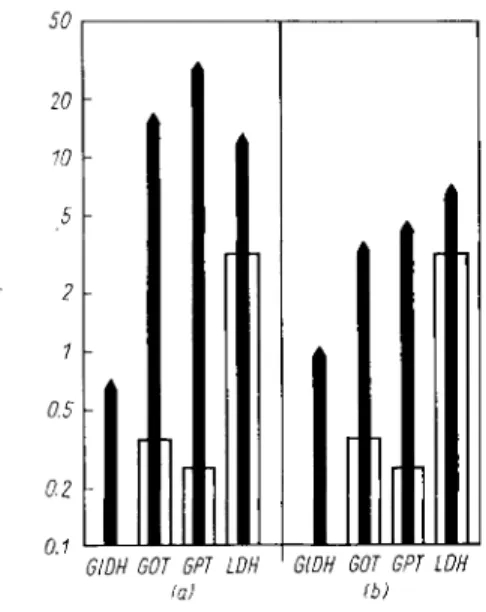
larity between the enzyme pattern of the serum and that of the diseased organ is more clearly seen in myocardial infarction than in hepatitis. Even though the qualitative relationship is plain in hepatitis (the ''liver-specifie" enzymes also occur in serum in hepatitis), the ratio of enzyme activities found in serum is partly changed in comparison to that occurring in the liver.
Biicher
23
* has called these differences in the ratio of enzyme activities distortion of the enzyme pattern. The chief causes are different locations of the enzymes in the c e l l
6 0 - 6 5
) , different rates of release of the enzymes, and inactivation of the enzymes at varying rates in the extracellular space. It is possible to distinguish approximately three types of enzymes according to their location in the cell
6 6 )
:
1. Cytoplasmic enzymes (e.g. lactic dehydrogenase)
2. Enzymes located only in the mitochondria (e.g. glutamic dehydrogenase)
3. Enzymes which occur in both cell compartments (e.g. glutamate-oxaloacetate transaminase and malic dehydrogenase)
Other differences due to different solubility, molecular weights and rates of diffusion may occur. It is possible that the rates of permeation through the cell membrane show corres
ponding differences. Perhaps the studies of Henley et al.**
1
) offer support for the above differences, since they showed that LDH, ICDH, GPT and G6P-DH *) in contrast to GOT could leave liver cells relatively easily, while glucose-6-phosphatase and G1DH could only be found in the surrounding medium after destruction of the cells. However, it must be expected that the enzymes of different tissues will have different rates of permeation 26,68).
After release from the cells the activity of the individual enzymes in serum decreases rapidly.
Any considerable elimination of the enzymes by the kidneys or with the bile plainly does not
occur29,68-71). Neither hepatectomy nor nephrectomy reduce the elimination of the enzy
m e s
7 2 7 3
) . There was also no difference in the rate of elimination when enzyme preparations were injected into the vena cava or portal vein
7 2
). A block of the reticuloendothelial system did not affect the disappearance of enzyme activity in serum
2 0
). To clarify the course of the extracellular inactivation of enzymes, numerous experiments have been undertaken injecting crystalline enzymes, purified enzyme preparations and crude homogenates of
*) For the meaning of the abbreviations, see under Table 2 (p. 658, 659).
6(» G. H. Hogeboom, J. biol. Chemistry 777, 847 [1950].
61) G. H. Hogeboom and W. C. Schneider, J. biol. Chemistry 186, 417 [1950].
62) W. C. Schneider and G. H. Hogeboom, J. biol. Chemistry 195, 161 [1952].
63) W. C. Schneider and G. H. Hogeboom, J. biol. Chemistry 204, 233 [1953].
64) C. DeDuve: Subcellular Particles. American Physiological Society, Washington 1959, p. 128.
H. Beaufay and C. DeDuve, Biochem. J. 73, 604 [1959].
66) A. Delbriick, H. Schimmassek, K. Bartsch and Th. Bucher, Biochem. Z. 331, 297 [1959].
67) K. S. Henley, O. Sorenson and H. M. Pollard, Nature [London] 184, 1400 [1959].
68) A. Jedlovsky, J. Kellen and K. Belay, Z. ges. inn. Med. Grenzgebiete 15, 884 [I960].
69) M. Chinsky, G. L. Shmagranoff and S. Sherry, J. Lab. clin. Med. 47, 108 [1956].
70) D. Amelung, H. D. Horn and E. Schroder, Klin. Wschr. 36, 963 [1958].
7D M. Dunn, J. Martins and K. Reissman, J. Lab. clin. Med. 51, 259 [1958].
72) p. E. Strandjord, K. E. Thomas and L. P. White, Clin. Res. 7, 224 [1959].
73) G. Forster and E. Jenny, Helv. med. Acta 26, 673 [1959].
658 Section C: Measurement of Enzyme Activity
Table 2a. Enzyme patterns of human tissue (E. Schmidt and F. W. Schmidt, Klin. Wschr. 38, 957 [I960])
3 10
k
o
2 10"
>.
' >
o
t
102N
C c
7tf 1 Leber
2 Pankreas
3 Niere Rinde
4 Niere Mark
5 Lunge
6 Grollhirn
Rinde 1 Grofthirn
Mark
8
Kleinhirn 9 Magen Mucosa
GAPDH
L DH
MDH LDH
MDH GAPDH
LDH
GAPDHMDH
LDH PK
GAPDHMDH L DH
PK MDH LDH
MDH GAPDH ,MDH LDH nu
GAPDH MDH
LDH GAPDH M DH
PK
GOT GOT GAPDH
LDH
GAPDH GOT GIDH SDH ICDH ADH GPT
HK PK
4
PK GOT
PK PK
EN ALD
HK
GOT EN
GIDH EN
GOT PK
EN
PK
GDH
GOT
GIDH ICDH GIDH
GIDH ICDH
EN
ALD PFA
EN ICDH
SDH GPT FN HK
ALD GDH
EN
GIDH
1CDH
HK
HK
GIDH
SDH
ICDH MD™" EN
IF GOT ICDH
IF
ICDH HK SDH
ALD
ALD
GIDH
""AM™"
PGDH ZF
GIDH m
HK ZF ZF PGDH PFA
ALU
GPT SDH ADH
PGDH GPT PFA SDH
GDH ZF
PGDH HK
K DH HK
SDH
GPT ADH IF SDH
PFA ADH PGDH
ZF PGDH GDH
PFA GDH PFA
PGDH ZF PFA GPT
PGDH
ALD GDH ADH ADH PFA GPT GDH ADH ADH GPT GDH ADH PFA GDH 70
J
10'
10 1 Liver
2 Pancreas 3 Kidney cortex
4 Kidney medulla 5 Lung
6 Cerebral cortex
7 Cerebral medulla 8 Cerebellum 9 Gastric mucosa A D H = Alcohol dehydrogenase
A L D = Aldolase E N = Enolase G A P D H = Glyceraldehyde-
3-phosphate dehydrogenase
H K = Hexokinase
I C D H = Isocitric dehydrogenase L D H = Lactic dehydrogenase M D H = Malic dehydrogenase P F A = Phosphofructoaldolase
*) A Biicher unit is the amount of enzyme in a 1 ml. assay mixture, which changes the optical density at 366 mu. by 0.100 in 100 s e c ; this corresponds to the conversion of 1.09 [xmoles sub
strate/hour (see p. 33).
I. a In Medicine 659
Table 2 b. Enzyme patterns of human tissue (continued from Table 2 a)
o
PQ C
10'
10"
10' 1 Magen- Muskul.
2 Uterus- Muskul.
3 Skelett- Muskel
4 Herz- Muskel
MDH 5 Fett- Gewebe
6 Lymph- knot en
7 Erythro- cyten 10
8 Sarkom
LDH
GAPDH
LDH
GAPDH
MDH LDH
GAPDH GAPDH
L DH
MDH
GAPDH
PK GAPDH LDH
MDH
LDH MDH
GOT
GAPDH MDH MDH
PK
GAPDH MDH
LDH
PK
PK ALD GOT
EN
PK GOT
G DH
IF ALD
ICDH
PK GPT PGDH
PK
EN IF PGDH
EN HK
IF
EN
GOT ICDH EN GIDH GOT
HK
EN PK HK
ALD ALD ICDH ALDICDH
HK
EN
GOT
HK ICDH AID
ICDH
HK G pJ
GDH
ALD G pT
EN
HK
GIDH
HK ICDH
ALD GIDH
IF
GOT
ICDH
GPT
GOT PGDH
GIDH GPT GIDH
IF GIDH IF
GPT PFA
GDH ADH
PGDH
SDH PGDH
GIDH
SDH ADH GPT PFA GDHGP1
ADH
SDH PFA GDH PGDH
ADH
SDH ADH IF PGDH
SDH PFA IF PGDH
ADH
SDH PFA PFA GDH PFA GDH ADH GIDH
\PFA GDH
I ADH
10 I
J
Gastric muscle Uterine muscle Skeletal muscle
10
h
10
J
10
1
•10 4 Heart muscle
5 Adipose tissue 6 Lymph glands G D H
G I D H G O T G P T
Glycerol-1-phosphate dehydrogenase P G D H (a-Glycerophosphate dehydrogenase) P K
Glutamic dehydrogenase S D H Glutamate-oxaloacetate transaminase Z F
Glutamate-pyruvate transaminase
7 Erythrocytes 1 0
11
8 Sarcoma
6-Phosphogluconic dehydrogenase Pyruvic kinase
Sorbitol dehydrogenase
Glucose-6-phosphate dehydrogenase (Zwischenferment)
660 Section C: Measurement of Enzyme Activity
organs20,50,70,71,74-79). According to Dunn et al.
1
^ the decrease of enzyme activity occurs in two phases: during the first rapid phase the activity in the lymph increases. After this equa
lization of concentration the activity slowly decreases in the second phase.
The rate of equilibration with the extracellular compartments
7 0
) and the rate of actual eli
mination of the enzymes varies from species to species and from enzyme to enzyme. While equilibrium in the distribution of transaminases in rabbits is reached in 70 to 80 seconds
8 0
), the equilibrium is not reached in the dog before 6 to 8 hours7 1
). It has been calculated from clinical data that the value for man is about 12 hours. According to Amelung*® the elimina
tion time for half of the liberated LDH in the rabbit is 150 minutes and for GPT is 308 minu
tes. In the dog the value for GOT is 12 hours and for GPT 20 hours
8
i>
8
2). The activity of LDH returns to normal after 6 hours in the dog, while that of ICDH has already returned to normal after 60—90 minutes
7 2
). The rate of elimination of enzymes in the human organism can be estimated from the decrease of enzyme activity in serum after acute organ damage of limited duration (e.g. selected cases of myocardial infarction, acute toxic liver damage). Wrob
lewski et al.
1083
) found half-lives of between 40 and 60 hours for GOT in myocardial infarc
tion. These values correspond to the data of Amelung*® in toxic liver damage (57 hours) and calculations of the authors (55 hours). The half-life time of GPT was found to be 78 to 88 hours. The half-life time of LDH (about 52 hours) is about as great as the half-life time of GOT. Approximately the same half-lives for LDH and GOT are also found in rabbit.
Which processes are responsible for the disappearance of enzyme activities after they have been evenly distributed is not known. The increase in the Michaelis constant of the MDH found in serum after myocardial infarction or in acute hepatitis is similar to that which occurs when the enzyme is aged in vitro
S4
K This suggests that the enzyme inactivation occurs within the blood vessels.
The distortion of the enzyme pattern of a particular organ on the passage of the enzymes into the serum depends on the course and severity of the cell damage. Severe cell damage of acute onset leads to rapid break-down of the cell membranes. The distortion of the enzyme pattern due to the different rates of inactivation of the enzymes can be avoided by early determination of the enzyme activities. On the other hand, in subliminal damage and slowly occurring pathological processes, the enzymes present initially in the serum are mainly cytoplasmic enzymes and subsequently different rates of inactivation may make the enzyme pattern unrecognizable.
The dependence of the amount of distortion of the enzyme pattern on the course of the disease is illustrated in Fig. 2 by the examples from myocardial infarction and hepatitis. In
74
> /. A. Sibley and G. A. Fleisher, Proc. Staff Meetings M a y o Clin. 29, 591 [1954].
7 5
> C. Huggins, Harvey Lectures 42, 148 [1947].
76
> /. A. Sibley and A. L. Lehninger, J. nat. Cancer Inst. 9, 303 [1949].
77
) F. Wroblewski and / . S. LaDue, Proc. Soc. exp. Biol. Med. 90, 210 [1955].
7
8) S. K. Wolfson jr., J. A. Spencer, R. L. Sterkel and H. G. Williams-Ashman, A n n . N . Y. Acad.
Sci. 75, 260 [1958].
79
> /. /. Sampson, Progr. cardiovasc. Dis. 1, 187 [1958].
80) D. Amelung, Hoppe-Seylers physiol. Chem. 318, 219 [I960].
80 G. A. Fleisher and K. G. Wakim, Proc. Staff Meetings Mayo Clin. 31, 640 [1956].
82) H. Reichard, J. Lab. clin. Med. 53, 417 [1959].
83) / . S. LaDue and F. Wroblewski, Circulation 15, 324 [1957].
84) E. Schmidt and F. W. Schmidt, Klin. Wschr. 38, 810 [I960].
2 A 2-1 2-\ 2-
1 1 1 L. 1 1 1 1 L I 1 I I !_ I I 1 | i_
1 2 3 k 1 2 3 k 1 2 3 k 1 2 3 k
faJ (b) (o (d) Fig. 3. Isoenzymes of lactic dehydrogenase (a) in serum in hepatitis, (b) in normal liver, (c) in normal
heart muscle, (d) in serum after myocardial infarction.
A . Separation on D E A E - c e l l u l o s e
1 1 7)
C. Dependence of the total L D H activity on the pyruvate concentration
8 5
>
Ordinate: Units/ml. eluate Ordinates: (a) and (d) Bucher units/ml. serum Abscissa: Number of fractions (b) and (c) 1 0
3
Bucher units/g. tissue B. Electrophoreticseparation
2 2
> Abscissae: 10~
3
M pyruvate Abscissa: % of the total activity
662 Section C: Measurement of Enzyme Activity
the acute ischaemia of the infarction the enzyme pattern in serum is remarkably similar to that of the heart muscle
5 7
,8 5
), although even in this case the ratios of the enzyme activities can be displaced by the unusually rapid inactivation of some enzymes (e.g. ICDH). In contrast, more marked changes in the ratios of the enzyme activities are found in the comparatively slower developing of hepatitis.
Enzyme patterns characteristic of a particular disease are found in serum, because these patterns are influenced by the changes in the enzyme patterns of the affected organs due the course and kind of disease.
The best known example of distortion is the change of the ratio of GOT/GPT in serum in acute hepatitis
8 6
,8 7 )
. Although in liver the activity of GOT is about 30% higher than that of GPT, in serum in hepatitis a larger proportion of GPT is regularly found. The faster rate of elimination of GOT is not sufficient (calculated on the basis of the accepted values) to explain the higher activity of GPT in serum. The higher activity occurs even at the start of the disease. The reversal of the ratio GOT/GPT in acute hepatitis is only exolained if the different intracellular location of the two transaminases is taken into account (about 40 % of the GOT is difficult to extract and is located in the mitochondrial compartment of the cell) and it is assumed that the two enzymes appear in the serum in the same proportions as they occur in the cytoplasm. This hypothesis is supported by the fact that the GOT occurring in serum has been shown to be cytoplasmic GOT by its Michaelis constant for a-oxoglutarate, and that as a rule only a relatively small increase of the mitochondrial GIDH is observed in serum.
However, in chronic diseases of the liver the ratio of activity of the two transaminases is a reflection of the conditions prevailing in the liver. Furthermore, since in this case in compari
son to the relatively small increase in the transaminases a much greater increase of GIDH in serum is observed, and the Michaelis constant of the GOT in serum corresponds to that of a mixture of cytoplasmic and mitochondrial GOT, it appears that in chronic liver diseases all compartments of the cell contribute to the enzymes found in the serum. When the pattern of the enzymes approximates to that of the liver, particularly severe liver damage is indicated (even to the extent of destruction of the individual cells) although the total damage to the organ as measured by the small increase of enzyme activity, would appear to be lower than that in acute hepatitis
5 8
).With larger increases of enzyme activity in serum the distortion is usually not sufficient to completely obliterate the similarity between the enzyme pattern in serum and that in the damaged organ. It is thus possible to associate the disease with a particular organ from the proportion of the activities of typical enzymes in the serum (usually it is sufficient to assay the activity of between 2 and 4 enzymes). Although this is usually only of theoretical interest, the possibility of estimating the extent of the participation of several organs during the course of a disease is of importance
5 7
,5 9
). However, such a differentiation is only possible with large increases of enzyme activity in serum. The very high sensitivity of the optical assay methods for enzymes (10~
10
moles enzyme in 0.1 ml. serum) can therefore not be fully exploited.
85) S.Linde, Scand. J. clin. Lab. Invest. 10, 303 [1958].
86) F. DeRitis, M. Coltorti and G. Giusti, Boll. Soc. ital. Biol. sperim. 31, 5 [1955].
87) F. DeRitis, M. Coltorti and G. Giusti, Minerva Med. /, 1 [1956].
L a In Medicine 663
The method of choice would be the assay of enzymes specific for particular organs. Largely specific to liver are *> SDH88-9D PFA52,73,92,93)
5
OCT94-98), A D H " ) . The changes in activity of creatine phosphokinase should be characteristic of myocardial infarction1 0 0
). However, either the activity of these enzymes in the organ is comparatively low or they are particularly quickly eliminated from the serum, so that their activity in serum has already returned to normal, when, for example, the transaminases still show significantly raised values.
4. Isoenzymes and their properties
Electrophoretic, chromatographic, kinetic and immunological methods have initiated further means of characterizing enzymes. It has been shown that enzymes with the same catalytic function, but originating from different organs or even different cell compartments of the same organ can be separated into different enzyme proteins, which have the same specificity.
Markert and Moller
10
) proposed the term "isoenzyme" for these heterogenous enzyme proteins. So far heterogenity of**) LDH21-23,102-120), MDH66,84,i07,i2i,i22)
?
ICDH122), G6P-DH122)? GDH122), enolase 123), GOT58.124), cytochrome c™K LAP126), alkaline*) O C T = ornithine carbamyl transferase. For the meaning of the other abbreviations, see under Table 2 (p. 658, 659).
**) L A P = leucine aminopeptidase. For the meaning of the other abbreviations, see under Table 2 (p. 658, 659).
88) u. Gerlach, Klin. Wschr. 37, 93 [1959].
89) H. Wiist and H. Schon, Klin. Wschr. 39, 280 [1961].
90) W. Brecht and /. KUnkele, Klin. Wschr. 38, 936 [I960].
9 D H. Siidhof, P. K. Riegel, H. Kuhlmann and D. Tietze, Dtsch. Arch. klin. Med. 206, 400 [I960].
92) H. P. Wolf, G. Forster and F. Leuthardt, Helv. physiol. Acta 15, C 44 [1957].
93) H. P. Wolf, G. Forster and F. Leuthardt, Gastroenterologia 87, 172 [1957].
94) W. E. C. Wacker, D. D. Ulmer and B. L. Vallee, N e w England J. Med. 255, 449 [1956].
95) H. Reichard, Scand. J. clin. Lab. Invest. 9, 103 [1957].
96) H. Reichard and P. Reichard, J. Lab. clin. Med. 52, 709 [1958].
97) L. Waitzkin, Ann. intern. Med. 49, 607 [1958].
98) R. Brown and S. Grisolia, J. Lab. clin. Med. 54, 617 [1959].
99) E. Schmidt, F. W. Schmidt and E. Wildhirt, Klin. Wschr. 37, 280 [1959].
100) / . C. Dreyfufi, G. Shapiro, J. Resnais and L. Scebat, Rev. franc. Etud. clin. biol. 5, 386 [I960].
101) C. L. Markert and F. Moller, Proc. nat. Acad. Sci. U S A 45, 753 [1959].
102) / . B. Neilands, Science [Washington] 115, 143 [1952].
103) Th. Wieland and G. Pfleiderer, Biochem. Z. 329, 112 [1957].
104) F. W. Sayre and B. R. Hill, Proc. Soc. exp. Biol. Med. 96, 695 [1957].
105) E. S. Vesell and A. G. Beam, Proc. Soc. exp. Biol. Med. 94, 96 [1957].
1
0 6
) /. Haupt and H. Giersberg, Naturwissenschaften 45, 268 [1958].
10
7
) E. S. Vesell and A. G. Beam, J. clin. Invest. 37, 672 [1958].
108) E. S. Vesell and A. G. Beam, Ann. N . Y. Acad. Sci. 75, 286 [1958].
109) B. R. Hill, A n n . N . Y. Acad. Sci. 75 304 [1958].
n o ) B. Hess, Ann. N . Y. Acad. Sci. 75, 292 [1958].
i n ) K. F. Gregory and F. Wroblewski, Clin. Res. 7, 295 [1959].
112) S. Futterman and / . H. Kinoshita, J. biol. Chemistry 234, 3174 [1959].
113) / . S. Nisselbaum and O. Bodansky, J. biol. Chemistry 234, 3276 [1959].
11
4
) N. O. Kaplan, M. M. Ciotti, M. Hamolski and R. E. Bieber, Science [Washington] 131, 392 [I960].
11
5
) P. G. W. Plagemann, K. F. Gregory and F. Wroblewski, J. biol. Chemistry 235, 2282 [I960], n o ) P. G. W. Plagemann, K. F. Gregory and F. Wroblewski, J. biol. Chemistry 235, 2288 [I960].
11
7
) B. Hess and S. I. Walter, Klin. Wschr. 38, 1080 [I960].
us) B. Hess and S. I. Walter, Klin. Wschr. 39, 213 [1961].
119
> G. Pfleiderer and E. D. Wachsmuth, 39, 352 [1961].
120) R. j . Wieme, Behringwerk-Mitt. 34, 27 [1958].
121) E. Schmidt and F. W. Schmidt, Klin. Wschr. 38, 957 [I960].
122) M. U. Tsao, Arch. Biochem. Biophysics 90, 234 [I960].
!23) B. G. Malmstrom, Arch. Biochem. Biophysics 70, 58 [1957].
124) E. Schmidt, F. W. Schmidt and Ch. Herfarth, Klin. Wschr. 40, 1133 [1962].
125) S. Palens and / . B. Neilands, Acta chem. scand. 4, 1024 [1951].
126
> O. D. Kowlessar, L. J. Haeffner and M. H. Sleisenger, J. clin. Invest. 39, 671 [I960].
664 Section C: Measurement of Enzyme Activity
phosphatase
1 2 7
>, acid phosphatase
1 2 8 )
, cholinesterase
1 2 8 - 1 3 0
), ribonuclease
1 3 1
,
1 3 2
), lyso- zyme
1 3 3 )
, pepsin1 3 4
), chymotrypsin
1 2 7
, xanthine oxidase
1 3 5
), caeruloplasmin
1 3 6
,
1 3 7
) has been demonstrated.
Electrophoretic separations of tissue homogenates have shown up to five bands of LDH of varying mobility. Each tissue shows a different distribution of activity in the individual enzyme fractions. Pfteiderer and Wachsmuth
n9)
distinguish three types of distribution.
In heart, brain and kidney, bands I and II, which migrate most rapidly to the anode, make up over 60% of the total activity. Band V, which is nearest to the anode, con
sists of 70—95% of the activity of smooth muscle, testicle, ovary, cartilage, lung, spleen, liver and epidermis. Isoenzymes of LDH which separate in an electric field have somewhat
2 4
) different properties1 1 6
.1 3 8
), whilst isoenzymes with the same mobility, bui originating from different organs have largely the same properties. The slower the rate of migration of the isoenzyme towards the anode, the smaller is the inhibition by sulphite i o n s
1 3 9 )
and the temperature coefficient of the reaction, and the lower the heat stability of the enzyme1 3 8
).Results comparable to the electrophoretic separation have been obtained by chromato
graphy of LDH on DEAE-cellulose
1 1 7 )
. Heart LDH which is strongly adsorbed on the column corresponds to the main band which migrates rapidly to the anode on electrophoresis (pH 7) whilst liver LDH is hardly adsorbed and on electrophoresis travels more slowly.
Similar agreement has been found for LDH from kidney and skeletal muscle.
Different substrate affinities also permit differentiation of enzymes with the same speci
ficity but of different origin. The majority of the studies so far carried out have been con
cerned with L D H
2 3
,
8 4
,
1 1 0
) . Differences have also been described for M D H
6 6
,
8 4
) and GOT
8 4
).
GOT has already been quoted as an example of where isoenzymes from different cell com
partments can have different substrate affinities.
Furthermore, it is also possible to distinguish the source of enzymes by their differ
ent reactions with coenzyme analogues
1 1 4
) or by serological methods. Precipitation and/or inhibition by antibodies has been demonstrated for*) phosphorylase
1 4 0
,1 4 1
)*) PHI = phosphohexoseisomerase; P G M = phosphoglucomutase. For the meaning of the other abbreviations, see under Table 2 (p. 658, 659).
!27) O. D. Kowlessar, J. H. Pert, L. J. Haeffner and M. H. Sleisenger, Proc. Soc. exp. Biol. Med. 100, 191 [1959].
!28) C. A. Dubbs, C. Vironia and / . M. Hilburn, Science [Washington] 131, 1529 [I960].
129) v. P. Whittaker, Physiol. Revs. 37, 312 [1951].
130) p. u. Angeletti, B. W. Moore and V. Suntzeff, Cancer Res. 20, 1592 [I960].
]
3 D A. A. Hakim, Arch. Biochem. Biophysics 83, 390 [1959].
!32) C. A. W. Hirs, W. H. Stein and A. S. Moore, J. Amer. chem. Soc. 73, 1893 [1951].
133) H. H. Tallau and W. H. Stein J. Amer. chem. Soc. 73, 2976 [1951].
!34) v. Desreux and R. M. Herriot, Nature [London] 144, 287 [1939].
1 3
5 ) E. Mitidieri, L. B. Ribeiro, O. A. Alfonso and G. G. Villela, Biochim. biophysica Acta 77, 587 [1955].
!36) L. Broman, Nature [London] 182, 1655 [1958].
137) R . Richterich, E. Gautier, H. Stillhart and E. Rossi, Biochem. Z. 331, 273 [1959].
!38) p. G. W. Plagemann, K. F. Gregory and F. Wroblewski, Biochem. Z. 334, 103 [1961].
139) Th. Wieland, G. Pfleiderer and F. Ortanderl, Biochem. Z. 331, 103 [1959].
140) \V. F. Henion and E. W. Sutherland, J. biol. Chemistry 224, All [1957].
H i ) M. N. Lipsett, R. B. Reisberg and O. Bodansky, Arch. Biochem. Biophysics 84, 171 [1959].
In Medicine 665
HK142), PHI 143), PGM 144), GAPDH 145,146), LDH H6. W7-15D and alkaline phospha
tase! 52-154).
In medical practice the organ-specific heterogeneity of enzymes is important, since it can also be demonstrated after the appearance of the enzymes in serum (Fig. 3). Analogous to the findings with electrophoretic separation of LDH isoenzymes from heart and liver, the highest activity in hepatitis serum was found in the slowest moving band, whilst in serum after myocardial infarction the highest activity was found in the rapidly moving bands 2 . 2 , 2 4 , 1 5 3 , 1 5 5 , 1 5 6 ) . it is note-worthy, that the changes in the pattern of isoenzymes in serum after myocardial infarction are still detectable when the total LDH activity in serum has returned to normal
1 5 7
). Similar agreement between the properties of the enzymes from various organs and the enzymes occurring in serum after damage to these organs is found by use of chromatographic methods
1 1 7
.1 1 8
). Hess11
® has published a very simple differential adsorption method for the separation of LDH isoenzymes from heart and liver on DEAE-cellulose.
It should not be overlooked that heart and liver occupy extreme positions with regard to the properties of their isoenzymes. With many other tissues the
t
great similarity of the isoenzymes1 1 9
) makes any accurate prediction about the extent of damage to a particular organ difficult. It is also difficult to classify isoenzymes by serological methods or on the basis of different substrate affinities. The significance of the differences found by the latter method for L D H 8 4 . i 0 7 , n o , i 5 8 )j M D H
8 4
) and G O T 5 8 ) i
n
serum in various diseases is obscured because the substrate affinities of the enzymes in the organs and in the serum are not always identical. Moreover, it has been shown that substrate affinity, particularly in the case of MDH, is not a constant value. Analogous to the in vitro ageing of the enzyme, the affinity probably begins to fall relatively rapidly after the release of the enzyme into the plasma8 4
).Despite their limitations, tests with a combination of the methods can give an astonish
ingly accurate picture about the source of individual enzymes in serum. It is to be hoped that simplification of the procedures may lead to their use in medical practice.
The differential adsorption method of Hess
11
® is a step towards a simplified technique (refer to p. 741).
142) /?. E. Miller, V. Z. Pasternak and M. G. Sevay, J. Bacteriol. 58 [1949].
143) M. N. Lipsett, R. B. Reisberg and O. Bodansky, Fed. Proc. 16, 212 [1957].
144) G. Bot and / . Redai, Naturwissenschaften 45, 467 [1958].
145) E. G. Krebs and V. A. Najjar, J. exp. Med. 88, 569 [1948].
146) p. Elddi, Acta physica Acad. Sci hung. 13, 199, 219, 233 [1958].
147) F. Kubowitz and P. Ott, Biochem. Z. 314, 94 [1943].
148) / . S. Nisselbaum and O. Bodansky, Fed. Proc. 18, 294 [1959].
149) / . 5. Nisselbaum and O. Bodansky, J. biol. Chemistry 236, 401 [1961].
150) / . S. Nisselbaum and O. Bodansky, J. biol. Chemistry 234, 5276 [1959].
15D / . S. Nisselbaum and O. Bodansky, J. biol. Chemistry 234, 3276 [1959].
152) M. Schlamowitz, J. biol. Chemistry 206, 369 [1954].
153) R. J. Wieme and L. Demeulenaere, Acta gastroenterologica Belg. 22, 69 [1959].
154) N. R. Keiding, Scand. J. clin. Lab. Invest. 11, 106 [1959].
155) H. Wiist, G. Berg, and H. Schon, Verh. dtsch. Ges. inn. Med. 66, 216 [I960].
156) R. J. Wieme, Clin. chim. Acta 4, 46 [1959].
153) F. Wroblewski, C. Ross and K. F. Gregory, N e w England J. Med. 263, 551 [I960].
158) D. Amelung, Habilitationsschrift, Dusseldorf 1959.
666 Section C: Measurement of Enzyme Activity
B) Diagnosis, Following the Course of Diseases, Control of Therapy, and Prognosis
I. Enzyme Activity in Serum
1. D i s e a s e s of the liver
The size of the human liver, the abundance of its enzymes (total activity D of LDH 12 x 10
6
units *), of GOT 5 x 106
units *>, of GPT and GIDH 3 x 10
6
units *>) and their variety have stimulated studies on the many enzymes which occur in serum in liver disease. A survey of the enzymes so far studied is given in Table 3.
Assay of the activity of the two transaminases and alkaline phosphatase have proved parti
cularly successful for the diagnosis and control of progress of liver diseases. With certain limitations, measurements of the activities of cholinesterase, sorbitol dehydrogenase, aldolase and glutamic dehydrogenase are also of value.
a) Acute hepatitis
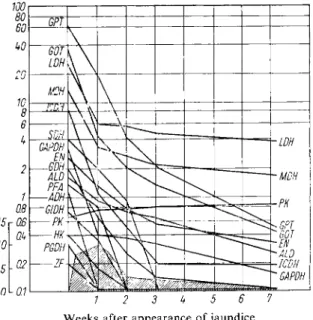
In the acute phase of viral hepatitis (Fig. 4) the activity of GOT in serum is increased 10 to 150-fold and that of GPT 20 to 200-fold. The quotient GOT/GPT (De Ritis^^)),^hioh
100
Weeks after appearance of jaundice
Fig. 4. Enzyme activity (curves and inner ordinate) and bilirubin level (shaded area and outer ordinate) in serum in acute hepatitis
1 7 4
>.
For the meaning of the abbreviations, see under Table 2 (p. 658, 659).
Inner ordinate: Bucher units/ml. serum Outer ordinate: m g . % bilirubin in serum
*) According to Bucher et al. For factors for conversion to other units, see p. 33; to obtain the data in Wroblewski units multiply by the factor 37.7.
i) E. Schmidt and F. W. Schmidt, Klin. Wschr. 38, 957 [I960].
Table 3. Enzymes in serum in liver diseases
Aldolase A L D 2-19) Isocitric dehydrogenase I C D H 10,47,55,56)
Alcohol dehydrogenase A D H 10,20) Lactic dehydrogenase L D H 6,9-11,13,57-62)
Amylase 21,22) Leucine aminopeptidase L A P 63-66)
Arginase 6,23) Lipoprotein lipase 67)
Quinine oxidase 24-26) Malic dehydrogenase M D H 10,11,16,62,68,69)
Cholesterol esterase 27-30) 5'-Nucleotidase 70,71)
Cholinesterase ChE 31-39) Ornithine carbamyl
Other esterases 9,40,41) transferase O C T 72-77)
Deoxyribonuclease D N a s e 108-110) Pepsinogen 78-80)
Enolase E N 10) Peptidases 81,82)
Fumarase F U M 42,43) Polyphenoloxidase 83,84)
p-Glucuronidase 44,45) Phosphofructoaldolase P F A 85-89)
Glucose-6-phosphatase 46) Phosphohexose isomerase PHI 4,7,69,90)
Glucose-6-phosphate dehydrogenase G 6 P - D H 10,47) Phosphogluconic
Glutamic dehydrogenase G I D H 10,11,48) dehydrogenase 6 - P G - D H 20,85,91)
Glyceraldehyde-3-phosphate G A P D H 10) Phosphoglucokinase 11)
dehydrogenase Phosphoglucomutase P G l u M 92-95)
a-Glycerophosphate dehydrogenase G D H 6,10,49) Alkaline phosphatase 4,16,96-107)
Glucuronyl transferase 50) Pyruvic kinase P K 10)
Glutathione reductase G R 51-53) Ribose-5-phosphate isomerase 111)
Hexokinase H K 10) Sorbitol dehydrogenase S D H 48,112,113)
a- Hydroxybuty ric 54) Transaminases 57,114-154)
dehydrogenase Transketolase 155)
2) F. H. Bruns, Biochem. Z. 325, 156 [1954].
3) F. H. Bruns and W. Puis, Klin. Wschr. 32, 656 [1954].
4) F. H. Bruns and W. Jakob, Klin. Wschr. 32, 1041 [1954]. 5
> /. A. Sibley and G. A. Fleisher, Proc. Staff Meetings Mayo Clin. 29, 591 [1954].
6
) B. Hess and E. Gehm, Klin. Wschr. 33, 91 [1955].
7
) F. Bruns and J. Neuhaus, Arch. Biochem. Biophysics 55, 588 [1955].
8) / . Eismdnn, Dtsch. med. J. 7, 204 [1956].
9) F. H. Bruns, Clin. chim. Acta 2, 257 [1957].
10) E. Schmidt, F. W. Schmidt and E. Wildhirt, Klin. Wschr. 36, 280 [1958].
I D A. Engelhardt-Golkel, R. Lobel, W. Seitz and /. Woller, Klin. Wschr. 36, 462 [1958].
12) L. G. Allgen, S. Izikowitz, B. Nauckhojf, J. B. Ordell and Salum, Science [Washington] 128, 304 [1958].
13) K. H. Goggel, Med. Klin. 933 [1958].
1
4
) A. Wielopolska and L. Wojciechowska, Przeglad Epidemiol. 12, 295 [1958].
is) U. Stawe, Z. Kinderheilkunde 81, 472, 675 [1958].
16) H. G. Klein, Wiener med. Wschr. 109, 179 [1959].


![Table 2a. Enzyme patterns of human tissue (E. Schmidt and F. W. Schmidt, Klin. Wschr. 38, 957 [I960]) 3 10 k o 2 10" >](https://thumb-eu.123doks.com/thumbv2/9dokorg/1153817.83136/8.702.84.615.158.828/table-enzyme-patterns-human-tissue-schmidt-schmidt-wschr.webp)