THE RHEOLOGY O F CELLULOSE DERIVATIVES Ε. B. Atkinson
I . T h e N a t u r e of Cellulose and its D e r i v a t i v e s 233
1. Chemical 3 2 3
2. P h y s i c a l 5 2 3
3. Plasticization and S o l u b i l i t y 7 2 3
I I . Fields of A p p l i c a t i o n 9 2 3
1. Plastics 9 2 3
2. Solutions and M e l t s 1 2 4
I I I . Irreversible F l o w B e h a v i o r 2 2 4
1. Solutions 2 2 4
2. M e l t s 4 2 4
3. Plastics 7 2 4
I V . E l a s t i c i t y and T o u g h n e s s 3 2 5
1. M o d e s of R e s p o n s e 3 2 5
2. Stress-Strain Curves 5 2 5
3. Creep 6 2 5
4. T o u g h n e s s 7 2 5
N o m e n c l a t u r e
T h e rheological properties of cellulose derivatives are best studied against the background of their structure, as revealed b y physical and chemical methods. A s y e t n o complete synthesis of the flow properties of any thermo- plastic can b e m a d e , given the chemical structure and molecular-weight distribution of the c o m p o n e n t p o l y m e r molecules, but a knowledge of the underlying structure of a plastic throws some light on the observed rheo- logical behavior, and often suggests ways in which this behavior can b e altered b y changes in the structural details.
I. The N a t u r e o f C e l l u l l o s e a n d its d e r i v a t i v e s1 1. CH E M I C A L
Cellulose occurs naturally as the main constituent of jute, c o t t o n , flax and other plant fibres. It is a polysaccharide based on the glucose ring, the cellulose molecule,
1 F o r fuller details of the chemistry, p h y s i c s , and t e c h n o l o g y of cellulose and its d e r i v a t i v e s , readers are referred t o Emil O t t , " C e l l u l o s e and Cellulose D e r i v a t i v e s . "
Interscience, N e w Y o r k , 1942.
233
234 Ε . Β . A T K I N S O N
C H2O H
Η O H
consisting of a large number of such units (i.e., over 1000) joined end t o end in a long flexible molecule. Starch is a chemically similar polysaccharide, but differs from cellulose in the details of its chain structure. Whereas the cellulose chain has the o x y g e n linkages alternating between positions a b o v e and b e l o w the planes of the glucoside rings (ß-glucoside linkage) in starch the oxygen linkages occur on the same side of each ring (a-glucoside link- age). T h e effect of this difference of structure on the physical properties of starch and cellulose is discussed b y Schoch.2
Cellulose may be dissolved in certain solvents (cuprammonium and xanthate solutions) and reprecipitated in acid media to give "regenerated cellulose" in the form of films or fibers, (cellophane, viscose rayon) processes which form the basis of very large industries. Here we shall be mainly con- cerned with chemical derivatives of cellulose which find application as thermoplastic molding materials such as
Other derivatives such as methyl cellulose and carboxymethyl cellulose are made as water-soluble thickening and suspending agents for foodstuffs, pharmaceutical products, detergents, etc.
Each glucoside ring in the cellulose chain has three hydroxyl groups—
one " p r i m a r y " and two " s e c o n d a r y " which m a y be esterified b y various organic and mineral acids or may also be "etherified" (ethyl and benzyl cellulose). T h e degree to \vhich the hydroxyl groups are substituted has a considerable effect upon the physical properties of the derivative, and a large part of the chemical technology of the production of cellulosic plastics is concerned with controlling the degree of substitution. In nitrocellulose the degree of substitution may be controlled b y the strength of the acids used, but in the case of cellulose acetate substitution must be allowed t o proceed to completion, i.e., to cellulose triacetate, if a homogeneous product
nitrocellulose or cellulose nitrate ) cellulose acetate and triacetate \ esters cellulose tripropionate J
cellulose aceto-butyrate " m i x e ethyl cellulose
1 ( < ethe]
benzyl cellulose / mixed ester
2 T . J. Schoch, J. Chem. Educ. 25, 626 (1948).
is t o b e obtained. T h e cellulose triacetate is then h y d r o l y z e d ( " b a c k hy- drolysis") t o reduce the acetyl content t o the required value, producing
" s e c o n d a r y " cellulose acetate. E t h y l cellulose is formed b y the reaction of ethyl chloride with soda cellulose (made b y treating cellulose with caustic soda solutions) and the degree of substitution m a y be varied b y a suitable choice of reaction conditions.
T h e degree of substitution of a cellulose derivative determines its degree of crystallinity, melting point, and solubility characteristics, and also such properties as chemical stability and water absorption. In native cellulose highly ordered regions occur k n o w n as "crystallites" or crystalline micelles, within which the molecular chains run parallel. X - r a y diffraction patterns indicate that the same three-dimensional order which occurs in ordinary crystals, prevails within the crystalline micelles, although there m a y b e transition regions between micelles which have a more random, less ordered structure ("fringe" or amorphous regions). Cellulose crystallites are held together b y strong " h y d r o g e n b o n d " forces between h y d r o x y l groups on neighboring chains—a feature which explains the insolubility of cellulose in c o m m o n solvents, as well as its high " m e l t i n g p o i n t . " A s more and more of these h y d r o x y l groups are substituted b y less strongly interacting chemical groups, (i.e., ordinary " p o l a r " groups such as acetyl radicles or nonpolar ethyl groups) the cohesion between the chains is reduced. If the substitut- ing groups are also b u l k y (butyrate or propionate) the cellulose chains cannot approach each other closely, and so the interaction forces are still further reduced. Since substitution usually takes place at r a n d o m along the chains, the resultant irregularity of the chain makes crystallization m o r e difficult, and solvent attack easier. T h u s chemical substitution of cellulose lowers the melting point, reduces crystallinity, and produces a material which can b e dissolved in c o m m o n organic solvents. W h e n the degree of substitution is increased t o such a degree that nearly all the available h y d r o x y l groups are substituted, the chain b e c o m e s regular again, the ease of crystallization and the melting point increases, and the solubility in or- ganic solvents decreases. T h u s for use as plastics and for applications as solutions, intermediate degrees of substitution are preferred, i.e., average number of h y d r o x y l groups substituted per glucoside residue
cellulose nitrate 2-2.26
2. PH Y S I C A L
X - r a y diffraction studies of cellulosic plastics and other evidence point t o a structure in which cellulose or substituted chains pass through b o t h ordered (or crystalline) regions and " a m o r p h o u s " regions within which n o
ethyl cellulose..
cellulose acetate
2.29-2.58 2.2-2.62
236 Ε . Β . A T K I N S O N
long-range order occurs. There is p r o b a b l y n o sharp dividing line between the crystalline and amorphous regions, but one m a y merge imperceptibly into the other. I t is assumed that an equilibrium m a y b e established b e - tween the t w o regions, equivalent t o a solid in equilibrium with its solution or melt. A t high temperatures the amorphous regions will grow at the ex- pense of the crystalline regions, b u t the nature of the equilibrium between the t w o " phases" is such that the progressive melting of crystallites will occur over a range of temperature. B y analogy with other polymers it is t o be expected that the degree of crystallinity, the size and m o r p h o l o g y of the crystalline regions and their subsequent melting points w o u l d b e determined b y the thermal history of the sample. A t l o w temperatures, especially b e l o w the ' second-order transition temperature" of the derivative, the amorphous regions b e c o m e extremely " v i s c o u s , " the m o b i l i t y of the chain segments passing through them, and especially their rotational mobility almost dis- appears. This shows itself as a loss of flexibility and toughness in the thermo- plastic. M o r e o v e r the equilibrium between the amorphous and crystalline regions will b e " frozen," and only extremely slow changes in the crystalline- amorphous ratio are t o be expected. In general the great length of p o l y m e r molecules makes their response t o changing conditions slow, since any con- siderable change in the relative positions of the molecule involves the statistical cooperation of a large number of chain segments in random Brownian m o v e m e n t , and when more chain segments are i n v o l v e d in any change, motions favorable t o the change occur less frequently. T h u s equilib- rium in the thermodynamic sense is p r o b a b l y the exception rather than the rule in solid thermoplastics.
T h e crystalline regions of a thermoplastic are mainly responsible for its cohesion, since they act as " cross links" between chemically unconnected chains and prevent large-scale relative m o v e m e n t s . T h e amorphous regions, however, are more readily deformed b y stress, especially a b o v e the transi- tion temperature, and largely determine the elastic modulus, resilience, and brittleness of the thermoplastic, whereas the crystalline regions contribute strength. Solvents and plasticizers are generally confined t o the amorphous regions and lower the transition temperature b y conferring greater mobility t o the disordered parts (there are exceptions t o this in the case of some cellulose nitrate—terpene systems in which the "plasticizer" seems t o form a " m i x e d crystal" with the cellulosic crystallites).3 B y " s w e l l i n g " the amorphous regions the plasticizer also upsets the equilibrium between the t w o regions and lowers the melting range of the crystallites.
A t high temperatures, when all crystallites have melted, cellulosics and other high polymers behave as very viscous and elastic liquids when sub-
3 Κ . H . M e y e r , " N a t u r a l and Synthetic H i g h P o l y m e r s , " p . 310. I n t e r s c i e n c e , N e w Y o r k , 1942.
j e c t e d t o small stresses. Under these conditions, and in solution, the viscous and elastic properties are greatly affected b y the chain length and b y the distribution of chain lengths in the bulk p o l y m e r . I n order that cellulose derivatives with useful flow and solution properties m a y b e m a d e it is usual t o " d e g r a d e " the cellulose t o a controlled degree in order t o reduce the molecular weight. Further degradation m a y occur during substitution reac- tions with the result that the final material has a considerably shorter chain length than the original cellulose and a v e r y b r o a d molecular weight dis- tribution.
Cellulosic chains differ from those of rubbers and v i n y l polymers in re- spect of their greater rigidity and linearity, caused b y the lack of free rota- tion a b o u t the chain linkages. R o t a t i o n through a limited arc is possible and thus gives the chain a slight flexibility. Nevertheless the average configura- tion of a cellulose molecule in solution would b e that of a rod bent at r a n d o m or a v e r y open coil c o m p a r e d with the m o r e tightly coiled configurations possible with materials having relatively free rotation a b o u t chain linkages.
T h i s restriction of free rotation and the rigidity of the chain as a whole re- sulting from the " r i g i d " glucoside rings is p r o b a b l y responsible for the greater t e n d e n c y of cellulosics t o crystallize, and for the higher melting points of their crystallites than those of v i n y l polymers with similar inter- chain cohesion. I n the solid state, t o o , the greater degree of mutual inter- penetration possible with chains of a m o r e extended configuration m a y give rise t o greater toughness and strength—a familiar feature of cellulosic plastics when c o m p a r e d with ordinary rigid vinyl materials. It is interesting t o note that the toughest vinyl-based p o l y m e r , p o l y v i n y l formal, has a structure which is
^ C H2
CH,4-CH C H - C H2 f C H X H - C H , j O
C H9
0>
C H ,
also likely t o h a v e its freedom of rotation a b o u t chain linkages restricted and t o h a v e a m o r e rigid chain structure.
3 . PL A S T I C I Z A T I O N A N D SO L U B I L I T Y
T h e mechanical properties and processing characteristics of cellulose der- ivations m a y b e modified b y the addition of various softening agents, i.e., solvents, plasticizers, "plasticizer extenders," and lubricants. A l t h o u g h large " e n t r o p y " effects play an important part in the t h e r m o d y n a m i c s of the solution of polymers, the factors w h i c h influence the solubility are
238 Ε . Β . A T K I N S O N
mainly those which operate in systems of l o w molecular weight. C o m p a t i - bility or mutual solubility between a p o l y m e r and a solvent or plasticizer is encouraged b y (a) similarity of chemical nature (i.e., similar t y p e and strength of V a n der W a a l s ' forces between neighboring molecules, which means the same " c o h e s i v e energy d e n s i t y " ; (6) specific interaction or c o m - plex formation between the plasticizer or solvent and the p o l y m e r . T h i s helps t o keep the chains separated and, b y shrouding the chain with less strongly interacting groups, prevents aggregation and precipitation.
Cellulose nitrate is readily soluble in ketones and organic esters b u t is insoluble in hydrocarbons and alcohols. H o w e v e r , mixtures of alcohols with esters or ketones have a high solvent power, as also has the mixture of ethyl alcohol and ether used t o m a k e d o p e s for film casting. C a m p h o r (which is a ketone) makes an excellent plasticizer whereas isoborneol (isomerically related t o c a m p h o r b u t an alcohol) is a p o o r plasticizer and of limited c o m - patibility. T h e specific effect of small structural changes can b e appreciated when the plasticizing effect of c a m p h o r and its isomer isofenchone (also a ketone) are c o m p a r e d .4 T h e " a c t i v a t i o n " of nonsolvents b y ketones and esters is p r o b a b l y due t o complex formation between the carbonyl group containing molecules and cellulose nitrate, the resulting " c o m p l e x " being soluble in alcohol. T h e properties of mixed solvent systems are of great interest in nitrocellulose or cellulose acetate lacquer t e c h n o l o g y .
Cellulose acetate has not the wide range of solvents available for cellulose nitrate. B o t h solvents and plasticizers need t o b e of a strongly polar nature (i.e., a high ratio of strongly polar groups t o nonpolar groups such as the methylene or methyl g r o u p ) . T h u s the only c o m m o n solvents are such ma- terials as acetone, ethyl acetate, and m e t h y l " C e l l o s o l v e , " and the c o m m o n plasticizers used are the methyl and ethyl esters of phthalic and phosphoric acid. M i x e d solvents of alcohols and nitroparaffins, analogous t o the alcohol- ester of ketone systems for nitrocellulose have high solvent power for cellulose acetate. Cellulose triacetate is only soluble in chlorinated h y d r o - carbons or mixtures of these with alcohols. E t h y l cellulose is soluble in aromatic h y d r o c a r b o n s and in their mixtures with alcohols (i.e., 80-20 toluene-ethyl alcohol mixtures).
M i n o r proportions of plasticizers are used with rigid ethyl cellulose and cellulose aceto-butyrate plastics t o modify the mechanical properties and p r o m o t e easy extrusion. Lubricants, which are materials of limited solu- bility, are often added t o p r o m o t e easy extrusion or injection molding.
W a x e s , stearic acid, etc., are generally used t o exude or b l o o m t o the surface of the material t o form a lubricating film and prevent adhesion t o the h o t metal of extrusion dies. (It should be noted that the term "internal lubri- c a n t " is occasionally used as a s y n o n y m for a plasticizer. True plasticizers
4 G . Swann, Ind. Chemist 25, 3 (1950).
act b y virtue of their solvent action, i.e., dispersing the p o l y m e r chains on a molecular scale, " l u b r i c a n t s " b y lowering the frictional forces between the plastic and metal surfaces.)
II. Fields o f A p p l i c a t i o n
T o appreciate the variety of rheological problems which arise during the manufacture of cellulose derivatives it is necessary t o consider the main fields in which they are used and the processes used during manufacture.
1. PL A S T I C S
Nitrocellulose plastics (Celluloid, xylonite, pyroxylin) :
T h e first synthetic thermoplastic material is still one of t h e m a i n cellulosic plastics, and is manufactured in the form of rod, sheet, tube, and cast film.
T h e other important cellulosic plastics—cellulose acetate, cellulose aceto- butyrate and ethyl cellulose—as well as being m a d e in these forms are used extensively as injection-molding powders. (Cellulose nitrate is n o t used as an injection-molding p o w d e r because of its limited stability at high tem- peratures.)
Nitrocellulose plastics are c o m p o u n d e d and fabricated b y a " s o l v e n t "
process, i.e., u p t o the final " s t o v i n g " of the half-finished sheets, tubes, etc., the plastic contains m o r e than 1 0 % of volatile materials (water and al- c o h o l ) , which enables the mixing and forming operations t o b e carried out at temperatures b e l o w 100° C . A l c o h o l - w e t nitrocotton is m i x e d with camphor, alcohol, and coloring matter in an enclosed mixer t o form a stiff dough. T h e d o u g h is filtered under high pressure t o r e m o v e dirt, and is milled on h o t rolls t o r e m o v e excess solvent. A t approximately 10 % solvent content it is rolled into " h i d e s . " These are pressed at moderate tempera- tures into thick blocks from which sheets are cut b y " s l i c i n g . " Sliced sheet can b e cut in thicknesses between 1 0 / 1 0 0 0 " and 3 ^ " . T h e sliced sheets are dried of their residual solvent b y hanging in heated chambers or " s t o v e s "
and are polished b y h o t pressing between polished metal plates in an hydraulic press. T h e b l o c k process of pressing and slicing lends itself readily t o the production of configurated patterns in the sheet, and artificial horn, ivory, and tortoiseshell effects can b e p r o d u c e d . R o d s and tubes are pro- duced b y extrusion from chipped-up rough sheet in screw extrusion m a - chines, or from cylinders of rolled-up sheets (dollies) in hydraulic extruders.
T h e material used contains 8 t o 1 0 % of alcohol enabling extrusion t o b e carried out at temperatures b e l o w 90° C . Higher temperatures are generally ruled out because of the danger of d e c o m p o s i t i o n and fire. R o d s and tubes produced thus are " s t o v e d " or seasoned in the same w a y as the sheet ma- terial t o r e m o v e residual solvent. T h e manufacture of the explosive cordite, consisting of a high nitrogen content (13 % ) nitrocellulose plasticized with
240 Ε . Β . A T K I N S O N
T A B L E I
Impact Strength Plastic Type Grade Flow Temp. 25° C. -φ° C Cellulose Nitrate (sheet) General Purpose
Transparent — 3 . 0 0.75
I m p r o v e d T o u g h n e s s
Transparent — 4 . 0 1.0
E t h y l Cellulose ( M o l d - General Purpose 2 143° C . 3 . 5 0 . 5 ing p o w d e r ) L o w temperature re- 6 143° C . 5.0 1.0
sistant
Cellulose A c e t o - B u t y r - General Purpose 3 149° C . 1.0 0.4 ate ( M o l d i n g p o w d e r ) I m p a c t resistant 8 127° C . 3.1 1.0 Cellulose A c e t a t e General Purpose 1 156° C . 0.8 0.3 ( M o l d i n g p o w d e r ) I m p a c t resistant 8 149° C . 1.7 0.4
nitroglycerin, is carried out b y similar processes. Cellulose acetate injection- molding chips or powders are m a d e b y t w o processes, the solvent or " w e t "
process and the dry process. T h e " w e t " process is fundamentally that described a b o v e for the production of nitrocellulose plastic extrusion chips.
Cellulose acetate molding powder, for instance, m a y be m a d e b y the wet process using a c e t o n e / a l c o h o l or methyl ethyl k e t o n e / a l c o h o l mixtures as solvents. Plasticizers are added t o the initial mixture, and the chipped-up sheet is dried t o r e m o v e residual solvent before use as an injection material.
Sliced sheet m a y b e produced from cellulose acetate b y the wet process as in the case of nitrocellulose.
T h e dry process consists of mixing the cellulose acetate p o w d e r or " f l a k e "
with colorants, plasticizers, stabilizers, etc., in a B a n b u r y mixer at high temperatures t o form a uniformly gelled mass which m a y b e rolled into sheet and cut into chips. A variation on this process uses screw extrusion machines t o homogenize the flake, plasticizer and color. T h e mixed material issues from the forming die of the extruder as a number of small diameter rods which m a y b e broken u p b y a rotary cutter into injection-molding granules of uniform size and shape.
All the cellulosic plastics with the exception of nitrocellulose are used t o make large numbers of articles b y means of the injection-molding process.
F o r the t e c h n o l o g y of this process readers are referred t o the chapter " T h e R h e o l o g y of M o l d i n g " b y C . E . B o y e r and R . S. Spencer, in V o l u m e I I I . C o l d molding granules are forced b y means of a hydraulic or pneumatically operated ram into a heated cylinder, usually with some form of torpedo or spreader at the h o t end. A s the granules m o v e with successive strokes of the
plunger into the h o t zone they b e c o m e softened and, in turn, issue from a nozzle at the end of the cylinder into a cold metal m o l d .
After allowing sufficient time for the material t o " s e t " in the m o l d b y cooling below the softening point, the m o l d is opened and the m o l d e d piece ejected. T h e m o l d then closes and the cycle is repeated. Cellulose acetate injection-molding p o w d e r s are supplied in a number of flow grades, i.e., soft, m e d i u m , m e d i u m hard, or hard, according t o the temperature required t o render t h e m sufficiently plastic t o b e injection m o l d e d . F l o w tests such as the " R o s s i - P e a k e "5 test are sometimes used t o characterize a temperature at which the material will have standard flow properties. T h e " R o s s i - P e a k e "
temperature of cellulose acetate molding powders m a y v a r y from 130 t o 170° C . according t o the flow grade. T h e flow grade m a y b e varied b y changing the degree of acetylation and viscosity of the original " f l a k e " or the plasticizer content. A b s o r b e d moisture in the injection p o w d e r has a considerable effect o n the flow properties, in effect acting as a plasticizer, and also gives rise t o steam bubbles in the moldings. T h u s it is of great i m - portance b o t h in injection-molding practice and in experimental rheological w o r k on cellulose derivatives t o d r y the m o l d i n g p o w d e r carefully before use.
Cellulosic films, i.e., sheet b e l o w 1 0 / 1 0 0 0 in. in thickness are manu- factured b y spreading or casting a concentrated solution or " d o p e " o n t o a m o v i n g metal wheel or band. T h e surface of the band is usually coated with a layer of gelatin t o p r o v i d e a s m o o t h uniform surface from which the film m a y b e stripped, and m o v e s inside a large heated b o x . T h e conditions are arranged so that the film has lost m o s t of its solvent b y evaporation b y the time it has reached the point at which it will b e stripped off as a c o n - tinuous sheet. B y passing through further heated cabinets, the remaining solvent is r e m o v e d and the film w o u n d u p in a roll. T h e t e c h n o l o g y of this process is complex, since, in addition t o the rheological problems of mixing, filtering and c o n v e y i n g the d o p e t o the spreader, the effect of solvent c o m - position on the properties of the film must b e taken into a c c o u n t . T h e sol- vents used are recovered from the vapor-laden air from the casting b o x and seasoning cabinets, and efficient r e c o v e r y is a prime necessity in the e c o - n o m i c success of the process. Similar principles a p p l y t o the " d r y spinning"
of acetate rayon.
2 . SO L U T I O N S A N D ME L T S
Solutions of cellulose derivatives are used v e r y extensively as adhesives, paints, and other protective coatings. Cellulosic enamels are used ex- tensively for m o t o r vehicles, and for other metal articles. Nitro-cellulose solutions are used for coating textile materials (synthetic leathercloth) and for applying decorative finishes t o leather, paper, etc. B o t h cellulose acetate
5 F . E . P i e c h and W . E . G l o o r , Am. Soc. Testing Materials Bull. 151, 70-75 (1948).
2 4 2 Ε . Β . A T K I N S O N
and cellulose nitrate solutions are used as adhesives. T h e main technological problem in formulating cellulosic paints is t o reconcile the various require- ments, i.e., high solids content, short drying time, freedom from " b l u s h i n g , "
or other drying defects and g o o d brushing or spraying characteristics.
E t h y l cellulose plasticized with an excess of a high-boiling-point mineral oil is used as a " h o t d i p " protective coating for metal parts, i.e., cutting tools, ball races, etc. T h e part t o be protected is q u i c k l y d i p p e d in the m o l t e n composition at 1 6 0 t o 1 8 0 ° C . A chilled layer of the material forms a t o u g h elastic skin around the article, which is impervious t o water and moisture.
T h i s coating m a y b e readily peeled off when the article is required for use.
III. Irreversible B e h a v i o r Flow
1. SO L U T I O N S
F r o m early in their history, the viscosity of their solutions at a fixed concentration has been regarded as one of the m o s t important properties of cellulose derivatives. W h i l e it was clear that the solution viscosity was of importance in relation t o the rheological properties of adhesives, dopes, and enamels, it was soon recognized that the viscosity of, say, a 1 2 % solution could be used as an index of m a n y other properties of a particular batch of a cellulose ester or ether, such as its toughness, solubility characteristics, and extrusion and m o l d i n g behavior. T h e w o r k of Staudinger m a d e it evident that the viscosity of dilute solutions of linear polymers was related t o their average chain length, u p o n which m o s t of the useful properties of the bulk p o l y m e r depend. Measurement of the viscosity of dilute solutions, as a means of determining the molecular weight of polymers is a routine practice in all branches of the plastics industry and has a v e r y extensive literature. O n l y a brief outline of the main facts in relation t o cellulose de- rivatives will b e attempted here.
W h e n the viscosity of a cellulose derivative is measured at several con- centrations in the range 0 t o 2 % the results can b e expressed in the following f o r m :
V = i?o[l + (v)C + BC*] ( 1 ) Other equations have been p r o p o s e d b y various workers. T h e constant
(η) which determines the fractional viscosity increase of the solution at l o w concentrations resulting from the addition of p o l y m e r molecules is termed the "intrinsic v i s c o s i t y . " T h e intrinsic viscosity is found t o b e related t o the weight average molecular weight in the following manner:
(η) = KM" ( 2 )
T h e appropriate values of Κ for cellulose derivatives are discussed else- w h e r e .6
6 M o l e c u l a r Weights of Cellulose, a S y m p o s i u m , Ind. Eng. Chem. 45, 2483 (1953).
T h e o r y relates the exponent a t o the t y p e of configuration of the p o l y m e r chain in solution—whether a rigid rod, tight or loose coil, etc. It is almost certain that the same value of a would n o t a p p l y for any p o l y m e r through- o u t the whole molecular-weight range. F o r m o s t cellulose derivatives a is v e r y nearly equal t o 1. T h e constant Β is c o m m o n l y regarded as an index of the degree of interaction between solute molecules. F o r discussion of the significance of the slope of specific viscosity-concentration plots see H u g - gins,7 and Eirich and R i s e m a n .8
A t high concentrations and even at l o w concentrations when the m o l e c u - lar weight of the solute is high, deviations from N e w t o n i a n behavior occur, further complicating the measurements. Viscometers h a v e been constructed t o give varying rates of shear in order that the measured viscosity should be extrapolated t o its value at v e r y l o w shear rates (see ref. 6, p . 2489). A s the concentration is increased n o n - N e w t o n i a n behavior and elastic effects b e c o m e m o r e apparent. Eisenschitz9 has measured the relation b e t w e e n shear stress, r, and rate of shear, g, in this region and found that the results m a y b e fitted t o the following t y p e of formula
g = AT + Br* (3)
A t l o w shear stresses (r being small) the second term b e c o m e s negligible and the solution behaves as a N e w t o n i a n fluid. A t high shear stresses the ap- parent viscosity decreases with increasing shear stress. T h e quantity VA/Β has the dimensions of an elastic modulus, and a theory of H e n c k y10 relates this t o the " e v a n e s c e n t " elasticity of the solution. Certainly at these concentrations and shear stresses orientation and elastic effects b e c o m e evident, and give rise t o flow birefringence, "elastic r e c o i l , " and the set of p h e n o m e n a variously described as " s e c o n d a r y flow," " c r o s s elasticity," and the " W e i s s e n b e r g effect." I n cellulose derivative solutions these m a y b e associated with the partial alignment of the molecules in the flow field t o - gether with some elastic distortion of the average chain segment distribu- tion. T h e Weissenberg effect, i.e., the appearance of normal surface tractions in liquids subjected t o a simple shearing action, has been suggested t o be also an expression of evanescent elasticity. W e i s s e n b e r g11 has suggested the following relation between the tension acting along the stream lines pxx and the shear stress τ
7 M . L . Huggins, Am. Chem. Soc. 64, 2716 (1942).
8 F . Eirich and J. Riseman, J. Polymer Set. 4, 417 (1949).
9 R . Eisenschitz, Kolloid-Z. 64, 184 (1933); see also W . Philippoff and K . Hess, Z.
physik. Chem. B31, 237 (1936); K . Edelmann, Proc. 2nd Intern. Rheol. Congr., Ox- ford p p . 107-115 (1953).
1 0 H . H e n c k y , Ann. Physik. [5] 2 , 617 (1929).
1 1 K . Weissenberg, Rept. General Conf. Brit. Rheologists' Club p . 36 (1946).
244 Ε . Β . A T K I N S O N 2
Vxx = T
Q
( 4 )where G is the " e v a n e s c e n t " shear modulus of the liquid. Some experiments m a d e b y the author showed that solutions of nitrocellulose in cyclohexanone o b e y e d this relation (4) within experimental error. T h e apparatus used was a simple shearing disk viscometer with a device for measuring the excess hydrostatic pressure at the centre of the disk due t o the "strangling"
tendency of the circular streamlines, as well as the total shearing forces acting on the disk surface. T h u s the variation of the magnitude of the secondary forces and of the viscosity of the material could b e measured simultaneously. T h e solutions o b e y e d relations (3) and (4) within experi- mental error, and the magnitude of G in (4) was close in value t o VΆ /Β derived from ( 3 ) . T h i s suggests a connection between anomalous viscosity effects, elasticity, and the Weissenberg effect which could be profitably in- vestigated further.
2 . ME L T S
T h e rheological properties of ethyl cellulose " h o t d i p " compositions de- scribed earlier are essentially those of concentrated solutions when they are in the molten state (120 t o 180° C ) , i.e., they are liquids, but with anoma- lous viscosity. W i t h decreasing temperature, however, a rapid increase in viscosity occurs over a narrow temperature range and the material forms a soft elastic gel. T h i s gel b e c o m e s more rigid with decreasing temperature t o b e c o m e a t o u g h flexible c o m p o u n d at r o o m temperatures. T h e formation of gels with a virtual " y i e l d stress" below a fairly well-defined temperature is characteristic of solutions of crystallizable polymers such as cellulosics, p o l y v i n y l chloride, polyethylene, etc., as distinct from polystyrene, p o l y - vinyl acetate and other amorphous polymers which show less marked gelling properties in solution. T h e rheological properties of highly plasticized ethyl cellulose compositions in the gel range are also of interest since these have been used as " c e m e n t s " or " b i n d e r s " for cork-based flooring compositions, as a replacement for " b o d i e d " (i.e., partially polymerized) linseed oil.
T h e rheological properties of interest are as follows : a. Melt Viscosity and Its Variation with Temperature
T h e melt viscosity determines t o a large extent the thickness of the film adhering t o the article when dipped in the melt and allowed t o drain. T h e viscosity increases with the intrinsic viscosity (i.e., molecular weight) of the ethyl cellulose used, and decreases with increase in temperature or plas- ticizer content. A convenient m e t h o d of measuring the viscosity is t o use an Umstatter or other t y p e of rotational viscometer, electrically heated.
T h e c o m p o u n d m a y be melted directly in the viscometer and the viscosity
determined over a range of temperatures. T h e empirical m e t h o d of dipping a metal plate into the mixture, draining, and measuring the thickness of the adherent film has also been used.
6. Softening Point or Melting Point
Various m e t h o d s h a v e been used t o determine a temperature at which the material will flow continuously under small sustained stresses. T h e ring and ball softening point test as used for bitumens can b e used for this pur- pose. T h e specimen used is a small disk fitting into a metal ring. A steel ball, smaller than the internal diameter of the ring rests on the disk, and the whole assembly is heated at a constant rate in a liquid heating path.
T h e temperature is found at which the ball penetrates the disk and falls through the ring. T h i s temperature will clearly b e related t o the viscosity- temperature curve of the hot-dip c o m p o u n d and the rate of heating.
In Fig. 1 the viscosity-temperature curves of three ethyl cellulose hot-dip compositions obtained using an Umstatter viscometer. T h e corresponding ring-and-ball softening points TR will b e seen t o correspond t o viscosities of approximately 7 X 103 poises.
A s a test of the m a x i m u m storage temperature for articles with a hot-dip coating, the deformation of cylinders of the composition compressed in a parallel-plate plastometer has been used. In one case the test consists of
1 03 poise
100 1 1 0 1 2 0 1 3 0 1 4 0 1 5 0 1 6 0 1 7 0 1 8 0 Temperature, °C.
F I G . 1. V i s c o s i t y - t e m p e r a t u r e curves of 3 ethyl cellulose h o t - d i p c o m p o s i t i o n s .
246 Ε . Β . A T K I N S O N
loading a cylinder of the composition 0.5 in. in diameter and 0.5 in. high with a load of 5 kg. applied between parallel plates 3 in. in diameter, at a temperature of 71° C . A m a x i m u m deformation of 0.35 in. is allowed after 5 min. under load, corresponding t o a ' viscosity' of approximately 108 poises.
c. "Creep" or "Cold Flow" at Room Temperatures
A p a r t from such tests as for tensile strength, it is of importance t o meas- ure the deformation of the dip coating gels described a b o v e under small stresses applied for long periods of time, since in m a n y applications—e.g., in cork-filled floor coverings—the material m a y be subjected t o indentation stresses from furniture, and so on. A simple m e t h o d of measuring the cold- flow properties of these materials is t o hang a small weight at one end of a
" d u m b e l l " tensile-test specimen and measure, at intervals, the length b e - tween t w o gage marks on the parallel portion of the specimen. T h e creep curves of Fig. 2 were obtained in this w a y .
T h e rheological behavior over the whole range of temperature i n v o l v e d in the use and manufacture of ethyl cellulose melt compositions m a y be con- trolled b y the molecular weight and degree of substitution of the ethyl cellulose, b y the proportion and aromatic content of the mineral oil used, and b y the addition of cross-linking agents and fillers. These different agencies d o not have identical effects at all temperatures, and so the possi- bility occurs of judiciously blending their effects t o obtain the best all- round results.
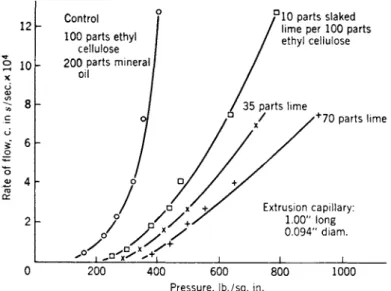
T h e action of calcium in the form of slaked lime on the flow properties of ethyl cellulose hot-dip compositions is peculiar and is illustrated in
) 1 I I I I
10 1 02 1 03 1 04 1 05
Time, seconds
F I G . 2. Creep curves for three E t h y l cellulose 'hot d i p ' c o m p o s i t i o n s . A , B , and C contain progressively smaller proportions of mineral oil plasticiser.
0 200 400 600 800 1000 Pressure, Ib./sq. in.
F I G. 3. A c t i o n of slaked lime o n the flow properties of ethyl cellulose h o t - d i p c o m p o s i t i o n s .
Fig. 3. T h e flow curves (extrusion pressure rate of flow curves for an ex- trusion p l a s t o m e t e r )l la were obtained at 90° C . i.e., in the " p l a s t i c " range and b e l o w the "ring-and-ball" softening point. T h e large increase in flow pres- sure obtained b y the addition of 1 0 % b y weight of lime suggests that the calcium h y d r o x i d e has a specific chemical effect on the ethyl cellulose, such as cross-linking residual O H groups between neighboring chains. Finely divided calcium carbonate had little effect on the flow curves b e y o n d that expected from an inert filler.
3. PL A S T I C S
Studies of the flow behavior of plastics in the range of temperature used in the fabrication of forms and articles (extrusion, calendering, and injec- tion and compression m o l d i n g ) are of o b v i o u s technical value. T h e y lead t o a fuller understanding of the mechanics of the process concerned, and they suggest h o w the process m a y be modified t o a c c o m m o d a t e less tractable thermoplastics or h o w the plastic must b e altered t o suit the process con- cerned. T h e exact behavior of polymers in the plastic range under even simple stresses is v e r y c o m p l i c a t e d12 and a study of the exact details of the flow of a plastic in the general case w o u l d not be practical for technical purposes. Fortunately sufficient information can be derived from relatively
1 1α Ε . B . Atkinson and H . A . N a n c a r r o w , Proc. 1st Intern. Rheol. Congr., Scheve- ningen Part I I , p p . 103-107 (1949).
12 W. F . O . Pollett, Proc. 2nd Intern. Rheol. Congr., Oxford pp. 85-90 (1953).
248 Ε . Β . A T K I N S O N
simple flow tests t o determine the approximate behavior of a p o l y m e r during extrusion and calendering operations.
Plasticity measurements have been used for v e r y m a n y years in the rub- ber industry as tests of extrusion of sheet calendering behavior, and the three main t y p e s of plastometer used in the rubber industry have been adapted for thermoplastic flow testing, i.e., the parallel-plate plastometer of Williams has been u s e d ;1 3-1 4»1 5 rotational viscometers ( M o o n e y ) b y Buchdahl, Nielsen, and M e r z ;1 6 extrusion plastometers b y N a s o n ,17 Spencer and D i l l o n ,18 Atkinson and N a n c a r r o w .l la T h e s e plastometers are all capable of giving information concerning the relation between the shear stress and the shear rate in the plastic c o m p o u n d . I t is also desirable for t h e m t o simulate the conditions of the process t h e y are used t o study or control.
E a c h t y p e has its advantages for the study of different aspects of a plastic's rheological behavior. F o r instance, the parallel-plate plastometer is ideally suited for measuring flow properties at v e r y l o w rates of shear and for measuring elastic r e c o v e r y . V e r y high rates of shear c o m p a r a b l e t o those in technical extrusion and calendering m a y b e obtained with the extrusion plastometer, while the rotational plastometer can b e used t o follow progres- sive changes in properties under a continued shearing action such as m a y occur in internal mixers, mills, and in extrusion screws.
T h e flow grades of cellulose acetate injection-molding powders are often specified b y the Rossi-Peake F l o w temperature5 ( A . S . T . M . D 569-43), this being the temperature at which the plastic will flow 1 in. in 2 min. into a
% in. tube under a pressure of 1500 l b . / s q . in. T h e Rossi-Peake tempera- tures of cellulose acetate molding powders v a r y from 130° C . for " s o f t "
grades t o 180° C . for hard, heat-resistant grades. T h i s test gives some indi- cation t o the molder of the temperature required in the cylinder of the in- jection machine—usually 20° C . higher than the Rossi-Peake temperature.
Practical experience of the injection process has shown that n o simple flow test will give c o m p l e t e picture of the behavior of the plastic in an in- jection-molding machine. T h i s is possibly due t o the fact that the tempera- ture of the material undergoes large changes during the molding cycle. I n the injection cylinder the plastic undergoes progressive heating and soften- ing until it reaches the nozzle at which it is of the consistency of a thick treacle. D u r i n g the injection stroke this material is forced into a cold or cool m o l d in a matter of a few seconds. A s the h o t plastic travels through the m o l d passages progressive cooling will occur and the situation will b e
1 3 W . M . Gearhart and W . D . K e n n e d y , Ind. Eng. Chem. 41, 695 (1949).
1 4 G . J. Dienes and H . F . K l e m m , Appl. Phys. 17, N o . 6, 458 (1946).
1 5 R . B u c h d a h l , Proc. 2nd Intern. Rheol. Congr.} Oxford p p . 116-122 (1953).
1 6 R . B u c h d a h l , L . E . Nielsen, and Ε . H . M e r z , </. Polymer Sei. 6, N o . 4, 403 (1951).
1 7 Η . K . N a s o n , / . Appl. Phys. 16, 338 (1945).
1 8 R . S. Spencer and Η . E . D i l l o n , Colloid Sei. 4, 241 (1949).
4
220eC. 2c 0 ( r
1 7 0eC .o
160°C.
120
• ο - Ι 40 Shear stress, (Pa/2/) lb./sq. in.
F I G . 4. R e l a t i o n between shear stress and rate of shear for cellulose acetate
" h a r d " grade injection m o l d i n g p o w d e r .
complicated b y the freezing of a cold skin of plastic on the metal surfaces.
M o l e c u l a r orientation resulting from the flow stresses occurs and the fin- ished m o l d i n g will exhibit the effects of this as a variation of strength properties in different directions in the m o l d i n g .
B y measuring the flow properties of plastics over a relatively wide t e m - perature range it is possible t o obtain a clearer picture of their injection behavior. A convenient m e t h o d is t o measure the pressure required t o ex- trude the material through a capillary of standard dimensions at a n u m b e r of different speeds, corresponding t o the shear rates obtained in the cylin- ders, m o l d s , and runners of injection molding machines. T h e pressure-rate of discharge data m a y b e analyzed t o give the relation between shear stress or " a p p a r e n t v i s c o s i t y " and rate of shear for the plastic at a n y tempera- t u r e .19 D a t a of this kind for a cellulose acetate m o l d i n g p o w d e r is shown in Fig. 4 and its behavior m a y b e taken as typical of m a n y thermoplastics.
A t high temperatures or l o w shear stresses the " f l o w c u r v e " relating shear stress and rate of shear is almost linear, whereas at lower tempera- tures and high shear stresses markedly n o n - N e w t o n i a n flow curves are obtained. It is thus impossible t o specify a viscosity at any temperature unless the shear stress or rate of shear is k n o w n (except perhaps at v e r y l o w stresses). W h e n it is required t o illustrate the temperature-dependence of
1 9 Ε . N . da C . A n d r a d e , " V i s c o s i t y and P l a s t i c i t y , " p . 56. Heifer, C a m b r i d g e , 1947; see also A . G . W a r d and E . L . E . W e s t b r o o k , Soc. Chem. Ind. 67, 389 (1948).
250 Ε . Β . A T K I N S O N
I I I I I I I I I I L 140 1 5 0 1 6 0 1 7 0 1 8 0 1 9 0 2 1 0 2 1 0 2 2 0 2 3 0
Temperature, eC .
F I G. 5. T e m p e r a t u r e variation in the flow properties of cellulose acetate and ethyl cellulose molding powders. A v e r a g e shear rate = 10 s e c .- 1.
the plastic flow properties of the system, a choice must b e m a d e between two m e t h o d s of presentation—whether t o plot the " v i s c o s i t y " measured at a fixed rate of shear or at a fixed shear stress against temperature. On theoretical grounds the latter m e t h o d is a more sound m e t h o d , but practical considerations make the former desirable. T h e shear rates at the same posi- tion in an injection-molding machine, extruder, or plastics calender are generally fixed b y the mechanics of the machine, while the shear stresses induced b y shearing the plastic v a r y with temperature. T h u s the shear stress or apparent viscosity measured at fixed shear rates are the m o r e use- ful quantities t o examine for temperature-dependence. Figure 5 illustrates the temperature variation in the flow properties of cellulose acetate and ethyl cellulose molding powders. T h e lower viscosity temperature coefficient of ethyl cellulose c o m p a r e d with cellulose acetate is v e r y evident, and argues a lower activation energy for the flow process in the former. Since the ethyl and acetyl groups are of comparable sizes it m a y b e assumed that the higher activation energy of flow in cellulose acetate is due t o the stronger secondary b o n d s formed b y interaction between the polar carbonyl groups in neigh- boring chains.
A d d i t i o n of plasticizers or solvents lowers the apparent viscosity of cellulosic compositions. Figure 6 shows the effect of progressive plasticizer additions on the flow properties of cellulose acetate c o m p o s i t i o n s .20
2 0 F o r other data on effect of plasticization on the flow properties of Cellulose acetate see V . Stannett and R . Whitfield, Plastics (London) 13, 15 (1949).
Ι Ο7
Ι Ο6
Ι Ο5
50 40
Parts of diethyl phthalate 100 cellulose acetate
140 150 160 170 180 190 200 210 Temperature, eC .
F I G . 6. Effect of progressive plasticizer additions on the flow properties of cellu- lose acetate c o m p o s i t i o n s . A v e r a g e shear rate = 10 s e c .- 1.
ω 4
175X.
Capillary 1.0" long 0.094" d i a m .
500 1000 1500
Pressure, lb./sq. in.
F I G . 7. Pressure rate of flow for a cellulose a c e t o - b u t y r a t e m o l d i n g c o m p o u n d .
Cellulose aceto-butyrate exhibits a curious p h e n o m e n o n when its flow properties are investigated b y extrusion methods. Figure 7 shows pressure- rate of flow curves for a cellulose aceto-butyrate molding c o m p o u n d . A s the extrusion speed is increased, the pressure d r o p through the capillary increases progressively at each temperature t o the point X. B e y o n d X a slight increase in speed causes a sharp and catastrophic rise in flow pressure, as though the plastic had suddenly " f r o z e n " in the capillary. T h e appear-
252 Ε . Β . A T K I N S O N
ance of the extruded rods also shows an abrupt change at X , changing from s m o o t h and shiny t o a dull, rough, and highly swollen extrusion. Cellulose aceto-butyrate has a relatively sharp softening range almost akin to a
" m e l t i n g p o i n t " compared with cellulose acetate, and this p h e n o m e n o n m a y be likened t o a "freezing" of the molten plastic under the influence of orientation induced b y flow through the capillary. A similar p h e n o m e n o n might occur if the viscosity of the plastic increased rapidly with the mean hydrostatic pressure, but the experimental results favor the " o r i e n t a t i o n "
theory.
Because of their thermal instability and inflammability, nitrocellulose plastics are not injection-molded and are only rolled, calendered, and ex- truded, as " w e t " or solvent-containing mixes at temperatures below 100° C . (usually 80 t o 90° C ) . A typical nitrocellulose extrusion material w o u l d contain 100 parts of nitrocellulose, 40 parts of c a m p h o r (as plasticizer) 10 parts of ethyl alcohol, and 2 parts of water (from "industrial" alcohol in- troduced at earlier stages), and would be extruded from a screw extruder having a die temperature of approximately 80° C . Figure 8 gives typical
" f l o w c u r v e s " of a nitrocellulose mix in this temperature range, and nearly approximate t o a " B i n g h a m b o d y " with a yield point. This yield point, however, is only apparent, since permanent flow will occur at m u c h smaller stresses. Elastic effects are v e r y marked and can give rise t o ripple-like markings and gross distortion of extruded rods and tubes. T h e temperature and extrusion speed range within which s m o o t h uniform extrusions can be produced is restricted compared with other plastics.
T h e flow curve alters with changes in the solution viscosity and nitrogen content of the nitrocellulose and proportions of alcohol, camphor, and water in the mixture.
0 5 0 0 1 0 0 0 1 5 0 0 2 0 0 0 Pressure, Ib./sq. in.
F I G . 8. F l o w of a nitrocellulose m i x (extrusion c o m p o u n d ) .
20 h
Ο) Ω - Ο) Τ3
0
ο 100 200 300
Time, seconds
F I G. 9. D e f o r m a t i o n and r e c o v e r y of a nitrocellulose extrusion c o m p o u n d . C o m - pression and r e c o v e r y at 9 0 ° C .
T h e elastic properties of nitrocellulose compositions m a y also be studied b y means of the parallel plate or " r e c o v e r y " plastometer or b y the extension of strips. E l e y and P e p p e r21 h a v e studied the viscoelastic properties of cordite in a similar w a y . Figure 9 shows deformation and recovery curves of a nitrocellulose extrusion c o m p o u n d in the form of an axially loaded cylinder, at 90° C . It will b e observed that a considerable proportion of the deformation is recoverable on removal of the applied load, and that delayed rubber-like elastic effects as well as viscous flow are present.
1. MO D E S O F RE S P O N S E
I n this final section a brief survey will b e m a d e of the mechanical proper- ties of cellulosic plastics at ordinary temperatures.
T h e response of solid high p o l y m e r materials t o stress is complex, and as y e t n o completely satisfactory molecular explanation of all the observed features has been made, b u t for convenience the following classification of the m o d e s of response can be m a d e .
Ordinary Elastic Straining
A n " o r d i n a r y " elastic deformation is always assumed t o be superimposed on other deformations resulting from stress. T h i s " o r d i n a r y " elasticity caused b y the bending of valency b o n d angles, straining of interchain v a n der W a a l s ' " b o n d s , " etc., is attained v e r y rapidly, is linear with stress and completely recoverable. A t v e r y l o w temperatures when configurational changes in the p o l y m e r chains cannot take place this mechanism is assumed
2 1 D . D . E l e y and D . C . Pepper, Trans. Faraday Soc. 43, 559 (1947).
I V . Elasticity a n d T o u g h n e s s
254 Ε . Β . A T K I N S O N
t o account for all the elastic deformation. T h e value of the elastic modulus of this process would lie between 1 011 and 1 012 d y n e s / s q . cms.
Configurational Elasticity
T h e elastic behavior of rubber and other polymers that are capable of large reversible extensions is ascribed t o the possibility of large deforma- tions in a network of randomly coiled high p o l y m e r chains when the chains can easily alter their configurations under thermal Brownian m o v e m e n t b y free rotation about single C — C b o n d s . T h e same process can occur in rigid or semirigid plastics, b u t at a m u c h slower rate. A rigid plastic is rigid (as is rubber at, say, —180° C.) b y virtue of the fact that configura- tional changes are greatly restricted b y interchain attractions, energy bar- riers restricting free rotation about the carbon-carbon b o n d s of the p o l y m e r chain " b a c k b o n e , " and crystallization. A n increase in temperature a b o v e the "second-order transition t e m p e r a t u r e " increases enormously the rate at which configurational changes can occur, and all linear polymers under these conditions display configurational elasticity.
A t r o o m temperatures the slow and progressive configurational changes under load give rise t o a time-dependent deformation or " c r e e p . " S o m e con- figurational changes can occur rapidly and so give rise t o an elastic deforma- tion which m a y be mistaken for " o r d i n a r y elasticity." T h e orientation times of the molecular processes i n v o l v e d cover m a n y decades, i.e., the elastic process has a distribution of orientation times. A t l o w stresses the configurational elastic deformations are reversible, i.e., on removal of the load the specimen slowly recovers its original shape. A n increase in tem- perature accelerates b o t h creep and recovery.
A t higher stresses the rate of configurational deformation increases v e r y rapidly with increase of stress, a phenomenon which gives rise t o "plastic yielding" and " n e c k i n g " in tensile-test specimens. This presumably occurs when the applied stress is sufficient t o o v e r c o m e the restraints which hinder configurational changes, e.g., rotational barriers, and van der W a a l s ' forces of crystallites. A b o v e the " y i e l d - p o i n t " strains of 100 t o 1 0 0 0 % are possible in polymers because of this mechanism. Nearly all of this deformation is recoverable b y heating a b o v e the second-order transition temperature.
Fracture
T o t a l failure of the specimen subjected t o a tensile stress m a y occur b y the propagation of a crack or tear across it. There is evidence that fracture is the culmination of progressive changes in the specimen brought about b y the applied stress. Similar changes, n o d o u b t , occur in specimens subjected t o alternating stresses or repeated blows, which ultimately fail b y cracking.
W h e n mechanical tests such as tensile-strength, fatigue, creep, and im-
p a c t tests are applied t o a plastic test specimen, the various m o d e s of re- sponse listed a b o v e will be i n v o l v e d in varying degrees, depending u p o n the stresses, rates of stressing, and the temperature. T h e " o r d i n a r y " elastic deformation is not in general a large c o m p o n e n t of the deformation and is n o t affected b y stress or the rate of stress application, so that the t y p e of behavior exhibited b y a plastic in any test is determined b y the interrelation of the other factors: configurational elasticity, structural changes, and frac- ture. T h e shape and extent of a stress-strain curve in a tensile test is deter- mined b y these three factors in competition. Toughness and high i m p a c t strength in a plastic is dependent u p o n an ability t o absorb energy at high strain rates without fracture, and H a w a r d22 has indicated h o w this is de- pendent u p o n the relation between the stress rate of creep curves and the stress-fracture time curves of a plastic.
T h e main factors which influence the tensile and yield strength of cellu- lose plastic compositions are molecular weight, degree and t y p e of substitu- tion, temperature, plasticizer content and relative humidity (since a b - sorbed water has plasticizing properties). Of particular interest is the effect of the chain length of the substituent groups, the tensile strength of triesters falling rapidly with increasing aliphatic chain length.23 Substituent groups and plasticizers b o t h have the effect of increasing the distance between the chains and so reducing the interchain forces and the t e n d e n c y t o crystallize.
B o t h factors lead t o a reduction in strength.
2 . ST R E S S -ST R A I N CU R V E S
Stress-strain curves of cellulose derivatives h a v e been published b y L a w t o n et al.2A This paper also gives a considerable a m o u n t of informa- tion on the variation of mechanical properties with temperature, humidity, rate of strain, etc. Another paper b y Carswell and N a s o n26 gives useful comparative data on the variation of the properties of several plastics (including vinyls, thermosetting materials, and cellulose derivatives) with temperature, humidity, weathering, and so on. Stress-strain curves in shear for cellulose acetate have been determined b y F i n d l e y26 and shear strengths h a v e been reported b y Schwartz and D u g g e r .27
T h e stress-strain curves and other mechanical properties described in the a b o v e papers fit into the general scheme of response described a b o v e . T h e stress-strain curves of cellulose acetate, cellulose nitrate and ethyl cellulose
2 2 R . N . H a w a r d , " S t r e n g t h of Plastics and G l a s s , " p p . 179-185. Interscience, N e w Y o r k , 1949.
2 3 S. E . Sheppard and P . T . N e w s o m e , J. Phys. Chem. 39, 143 (1935).
2 4 T . S. L a w t o n , T . S. Carswell, and H . K . N a s o n , Modern Plastics 22, 145 (1944).
2 5 T . S. Carswell and H . K . N a s o n , Modern Plastics 21, 125 (1944).
2 6 W . N . F i n d l e y , Modern Plastics 20, 99 (1943).
2 7 R . T . Schwartz and E . D u g g e r . Modern Plastics 21, 117 (1944).
2 5 6 Ε . Β . A T K I N S O N
plastic materials show an initial recoverable elastic response at l o w stresses, with noticeable departures from linearity at stresses approaching the
" y i e l d stress."
T h e " y i e l d stress" is n o t a sharply defined quantity when applied t o organic thermoplastics and (unlike, for instance, t o u g h metals) varies con- siderably with the rate of strain i m p o s e d during the tensile test. I n fact the " y i e l d stress" m a y b e taken as the stress at which the rate of creep of the specimen b e c o m e s equal t o the linear speed of the cross-head of the tensile testing machine. Yielding is often a c c o m p a n i e d b y " n e c k i n g " of the specimen because of the inevitable increase in stress caused b y a local reduction in area, and occasionally " s l i p b a n d s " will appear similar t o those found on metal specimens stressed b e y o n d their yield point. H o w e v e r , such slip bands are found t o lie at angles greater than 4 5 deg. t o the direc- tion of the tensile stress, which suggests that the yield stress in shear is reduced b y a hydrostatic tension.
B e y o n d the yield point a cellulosic plastic can b e " c o l d d r a w n " (often with necking) b y an extension of u p t o 5 0 % . H a w a r d28 has suggested that the high elastic extensibility of cellulose derivatives m a y b e limited b y the comparative straightness of the cellulose chains which does n o t allow m u c h extension b y " u n c o i l i n g . " Extensibility, especially two-dimensional ex- tensibility at ordinary and elevated temperatures is of considerable tech- nical importance, as it is essential for such forming operations as bending and punching, at r o o m temperatures and v a c u u m and " b l o w " molding, at elevated temperatures. T h e elongation at break as measured b y tensile tests on cellulosic plastic sheet materials increases in a general w a y with increase in temperature, until values of 1 0 0 t o 1 5 0 % are obtained in the blow-molding temperature range ( 1 1 0 t o 1 3 0 ° C . ) for cellulose acetate and nitrate sheets. E t h y l cellulose sheet has a higher elongation in this temperature range ( 1 5 0 t o 3 0 0 % ) b u t generally suffers from other diffi- culties resulting from its tendency t o stick t o h o t metals.
3 . CR E E P
W h e n subjected t o fixed loads, cellulose plastics, like m a n y other thermo- plastics, " c r e e p , " that is, they extend progressively with time. A s has al- ready been indicated, the rate of creep increases rapidly when the stresses approach the yield stress. Other investigations of the creep behavior of cellulosic p l a s t i c s2 8a h a v e been m a d e b y B u r n s ,29 C h a s m a n ,30 H a w a r d ,22 and
2 8 R . N . H a w a r d , Trans. Faraday Soc. 38, 394 (1942).
28a j?0T an excellent i n t r o d u c t i o n t o the nature and origin of creep in organic m a - terials (including viscose and acetate r a y o n ) and t o its scientific s t u d y the reader is referred t o " T h e Elastic and Creep Properties of Filamentous Materials and Other H i g h P o l y m e r s " b y H e r b e r t Leaderman (Textile F o u n d a t i o n , W a s h i n g t o n ) .
W a r d and M a r r i o t t .31 A n interesting feature of m u c h of this w o r k is that the creep curves obtained can v e r y often b e fitted t o an equation which is v e r y similar t o A n d r a d e ' s equation for the creep of metals:
Creep strain = at11* + bt
Under a constant load a thermoplastic material m a y eventually fail b y fracture. T h e time required for fracture t o take place varies with the stress applied, b e c o m i n g short at stresses approaching the breaking stress as measured in an ordinary tensile-strength test. T h e relation between applied stress and time t o rupture for cellulose derivatives has been discussed b y H a w a r d ,22 F i n d l e y ,32 and C h a s m a n .30 T h e r e is evidence that progressive irreversible changes take place under a constant stress which finally result in fracture, in a similar manner t o the fatigue damage caused b y alternating stresses. There seems t o b e a need for clarifying the connection between the effect of sustained and alternating stresses on fatigue, crazing, creep, d y - namic loss, and so o n .
4 . TO U G H N E S S
T o u g h n e s s and resistance t o shock in plastic materials are usually as- sessed b y impact-strength tests. T h e r e is a variety of these based on test specimens in the form of n o t c h e d or plain bars which m a y b e clamped at one end and struck at the other end ( I z o d test) or supported at each end and struck in the middle ( C h a r p y test). I n these tests the striker m a y b e a h e a v y pendulum or a falling weight. A n o t h e r t y p e of test used consists of dropping a weighted carriage or a steel ball on t o a plate or disk of plastic material which m a y b e clamped around its edge. T h e " i m p a c t strength"
is expressed either as (a) the m i n i m u m energy required t o fracture the specimen, or as (b) the energy absorbed from the striker in breaking the specimen, a quantity which is n o t necessarily the same as ( a ) . Opinions differ over the relative usefulness of these t w o m e t h o d s of testing and indeed over the relevance of " i m p a c t strengths" as a guide t o the toughness of a thermoplastic, since t w o kinds of i m p a c t test will often grade a series of thermoplastic materials in a different order of merit.
T h e nature of toughness and the meaning of impact-test results h a v e been discussed b y H a w a r d ,33 C o k e r and F i l o n ,34 and others. T h e i m p a c t strength
2 9 R . Burns, Modern Plastics 21, 111 (1943).
3 0 B . Chasman, Modern Plastics 21, 145 (1944).
3 1 A . G . W a r d and R . R . M a r r i o t t , / . Sei. Instr. 25, N o . 5, 147 (1948).
3 2 W . N . Findley, Modern Plastics 18, 83 (1941).
3 3 R . N . Haward, "Strength of Plasties and G l a s s , " p . 146. Cleaver-Hume Press, L o n d o n , Interscience, N e w Y o r k , 1949).
3 4 E . G . Coker and L . N . G . Filon, " A Treatise on P h o t o e l a s t i c i t y , " p . 595. C a m - bridge U n i v . Press., L o n d o n , and N e w Y o r k , 1931,