Thermodynamics of formation of b-cyclodextrin inclusion complexes with four series of surfactant homologs
Ma´ria Benk}o• Re´ka Tabajdi• Zolta´n Kira´ly
Received: 15 May 2012 / Accepted: 13 July 2012 / Published online: 21 August 2012 ÓAkade´miai Kiado´, Budapest, Hungary 2012
Abstract We report on a titration microcalorimetric study of the formation of b-cyclodextrin inclusion com- plexes with the members of series of anionic, cationic, and nonionic surfactant homologs containing even numbers of carbon atoms in the alkyl chains.n-Alkyltrimethylammo- nium bromides (CxTAB, x=6–16), sodium n-alkyl sul- fates (SCxS, x=6–12), sodium n-alkanesulfonates (SCxSN, x=6–12), and N,N-dimethylalkylamine-N-oxi- des (DCxAO, x=8–12) were selected for use. The stoi- chiometry, the binding constant, and the changes in enthalpy, entropy, and Gibbs free energy of the complex- ation reactions were determined at 298 K. It was found in each case that the complexation is favored by both the enthalpy and the entropy changes and that the thermody- namics of the process is affected far more strongly by the length of the alkyl chain than by the nature of the headgroup.
Keywords Surfactants b-Cyclodextrin Inclusion complexesIsothermal titration microcalorimetry Thermodynamics
Abbreviations
CxTAB n-Alkyltrimethylammonium bromide C6TAB n-Hexyltrimethylammonium bromide
C10TAB n-Decyltrimethylammonium bromide C12TAB n-Dodecyltrimethylammonium bromide C14TAB n-Tetradecyltrimethylammonium bromide C16TAB n-Hexadecyltrimethylammonium bromide SCxS Sodiumn-alkyl sulfate
SC6S Sodiumn-hexyl sulfate SC8S Sodiumn-octyl sulfate SC10S Sodiumn-decyl sulfate SC12S Sodiumn-dodecyl sulfate SCxSN Sodiumn-alkanesulfonate SC6SN Sodiumn-hexanesulfonate SC8SN Sodiumn-octanelsulfonate SC10SN Sodiumn-decanesulfonate SC12SN Sodiumn-dodecanesulfonate DCxAO Dimethylalkylamine-N-oxide DC8AO N,N-dimethyloctylamine-N-oxide DC10AO N,N-dimethyldecylamine-N-oxide DC12AO N,N-dimethyldodecylamine-N-oxide bCD b-cyclodextrin
S Surfactant
bCD-S Host–guest inclusion complex K Binding constant
[bCD-S] Concentration of inclusion complex [bCD] Concentration of free host compound [S] Concentration of free guest compound Qi Heat absorbed or evolved per injection DHi Molar heat of binding per injection Vo Volume of calorimeter vessel P The calorimeter power signal
N The stoichiometry of the complexation reaction DH The enthalpy of binding
DG The Gibbs free energy of the reaction TDS The entropy term of the reaction R The gas constant
T Temperature
M. Benk}oR. TabajdiZ. Kira´ly (&)
Department of Physical Chemistry and Materials Science, University of Szeged, Aradi Vt. 1, Szeged 6720, Hungary e-mail: zkiraly@chem.u-szeged.hu
Present Address:
M. Benk}o
Supramolecular and Nanostructured Materials Research Group of HAS, Department of Medical Chemistry, University of Szeged, Do´m te´r 8, Szeged 6720, Hungary DOI 10.1007/s10973-012-2603-0
Introduction
Native cyclodextrins (CDs) are composed of 6 to 8 (a-1,4)- linked a-D-glucopyranose units which form a rigid torus- shaped conformation with a central cavity ranging from 0.5 to 0.8 nm in diameter [1]. While the outer surface of the truncated-cone (or doughnut-shaped) CD molecule is hydrophilic, the inner surface is hydrophobic. As a con- sequence, CDs are water soluble and can accommodate poorly water-soluble compounds in their cavity, provided that the guest molecules fit into the interior of the host molecules geometrically. This molecular encapsulation is termed inclusion complex formation [2].
CDs, formed from starch in enzymatic reactions, are nontoxic, environmentally friendly materials which can be regarded as products of renewable energy sources, and they are therefore important compounds in green chemistry. The physical and chemical properties of CDs (the molecular size and shape; the cavity diameter; the number of high- energy water molecules in the cavity; their participation in a large number of chemical reactions, including function- alization; etc.) are well known and are available in the literature [3,4].
b-Cyclodextrin (bCD), an easily accessible low-priced material, is the most frequently used fine chemical among the native CDs. In general, bCD and its functionalized derivatives are hydrophilic and are capable of passing through biologic membranes. Structurally,bCD is the most stable CD. Moreover, its high stability affects the stability of the guest compound. For example, inclusion complex- ation with volatile materials hinders their volatility, and the complexes can be converted to, and stored in powder form.
Alternatively, the complexation reaction can be performed directly on the powder form.
bCD and its derivatives are of great interests as concerns industrial applications. As host materials, they are impor- tant components for drug formulations in the pharmaceu- tical industry [5–7] and they are also efficient solubilizers [8]. CDs are frequently used in biotechnology [9], the food industry [10], and waste water treatment [11].
Surfactant molecules mostly consist of a hydrophilic headgroup and a hydrophobic tail. Owing to their amphi- pathic properties, these compounds are widely used as detergents, wetting agents, foaming agents, emulsifiers, suspension stabilizers, and solubilizers [12, 13]. Besides their widespread applications in households and industry, surfactants are additionally of great academic interest. For example, because of their self-assembling properties, sur- factants are excellent model systems with which they mimic phospholipids as the major constituents of cell membranes in biologic systems [14].
The interactions between surfactants and CDs have attracted significant scientific and technological interest.
Surfactant molecules are ideal guest compounds for CDs as host materials. The hydrophobic part of the surfactant can be accommodated in the hydrophobic cavity of the CD ring, while the hydrophilic headgroup remains in the aqueous phase, but close to the rim of the CD ring [15–19]. The application of surfactants allows a systematic study of inclusion complex formation with CDs since both their hydrophobic and hydrophilic moieties may be systemati- cally changed, e.g., variation of the length of the alkyl chain with a fixed headgroup, or of the nature of the headgroup with a fixed alkyl chain length. Among the various experimental methods that exist for the study of inclusion complex for- mation, one of the most powerful methods is isotherm titration microcalorimetry [20]. The stoichiometry, the binding constant, and the changes in enthalpy, entropy, and Gibbs free energy can be determined over a wide range of temperature. We earlier reported on the thermodynamics of complexation ofbCD with cationic, anionic, and nonionic surfactants, each containing a dodecyl chain [16]. Mea- surements at 288–348 K revealed that the binding constant (K) decreased markedly with increasing temperature; the enthalpy change (DH) and the entropy term (TDS) were strongly temperature dependent but hardly affected by the nature of the headgroup; the change in the Gibbs free energy (DG) was rather small, in consequence of entropy–enthalpy compensation. In the present work, we extend that study and report on the thermodynamics of the interactions between bCD and the members of four series of surfactant homologs at a fixed temperature of 298 K.
Experimental
Materials
bCD was a product of Cyclolab Ltd, Budapest, Hungary. The powder contained 13.1 % water as determined using a Mettler-Toledo thermogravimetric apparatus. Knowledge of the water content was necessary for the accurate preparation of solutions with the desired concentrations. The cationic surfactants used were members of then-alkyltrimethylam- monium bromide homolog series (CxTAB). n-hexyltrime- thylammonium bromide (C6TAB) (98 %, Alfa Aesar), n-octyltrimethylammonium bromide (C8TAB) (97 %, Alfa Aesar), n-decyltrimethylammonium bromide (C10TAB) (98 %, Alfa Aesar),n-dodecyltrimethylammonium bromide (C12TAB) (99 %, Sigma),n-tetradecyltrimethylammonium bromide (C14TAB) (99 %, Sigma), and n-hexadecyl- trimethylammonium bromide (C16TAB) (99 %, Sigma). We applied two anionic surfactant homolog series: sodium n- alkyl sulfates (SCxS) and sodium n-alkylsulfonates (SCxSN). The following even-carbon-number members were used: sodiumn-hexyl sulfate (SC6S) (99 %, Lancaster),
sodiumn-octyl sulfate (SC8S) (99 %, Alfa Aesar), sodiumn- decyl sulfate (SC10S) (99 %, Alfa Aesar), sodiumn-dodecyl sulfate (SC12S) (99 %, Sigma-Aldrich) and sodiumn-hex- anesulfonate (SC6SN) (99 %, Alfa Aesar), sodium n-oc- tanesulfonate (SC8SN) (99 %, Alfa Aesar), sodium n- decanesulfonate (SC10SN) (99 %, Alfa Aesar), sodium n- dodecanesulfonate (SC12SN) (99 %, Alfa Aesar). N,N- dimethylalkylamine-N-oxides (DCxAO) at pH=8: N,N- dimethyloctylamine-N-oxide (DC8AO) (98 %, Fluka),N,N- dimethyldecylamine-N-oxide (DC10AO) (in 0.1 M solution, Fluka), andN,N-dimethyldodecylamine-N-oxide (DC12AO) (in 0.1 M solution, Fluka).
All solutions for the calorimetric experiments were prepared using Milli-Q water. The concentrations of the solutions are listed in Table1.
Microcalorimetric measurements
The experimentation was the same as described previously [16]. The heats of complexations at 298 K were measured using a Microcal VP-ITC power compensation microcal- orimeter [27,28]. The calorimeter vessel was loaded with bCD solution (1.4163 mL cell volume) and titrated with the surfactant solution in a 290-lL continuously stirring syringe. Twenty-nine 10-lL aliquots were injected into the sample cell at in intervals of 5 min. For each injection, the heat evolved was continuously recorded. The enthalpogram (calorimeter power signal vs. time) was composed of the
series of calorimeter peaks of the 29 sequential injections.
Data analysis was carried by the Microcal OriginÒ 7 software. The experimental error of the determination of the reaction enthalpies was less than 3.0 %.
Thermodynamic analysis
The 1:1 complexation reaction of bCD with surfactant S can be described by the following equation [16,27,28]:
bCDþS,K bCDS ð1Þ
wherebCD-S denotes the host–guest inclusion complex.
The binding constant is defined as K¼ ½bCDS
½bCD ½ S ð2Þ
where [bCD-S], [bCD], and [S] are the concentrations (in mol dm-3) of the inclusion complex, the host, and the guest species, respectively.
The complexation reaction is monitored by the calo- rimeter as
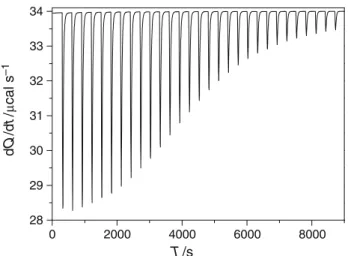
Qi¼DHiV0½bCDSi ð3Þ where Qi is the heat evolved (or absorbed), DHi is the molar heat of binding per injection, andVois the volume of the calorimeter vessel. A typical enthalpogram is presented in Fig. 1.
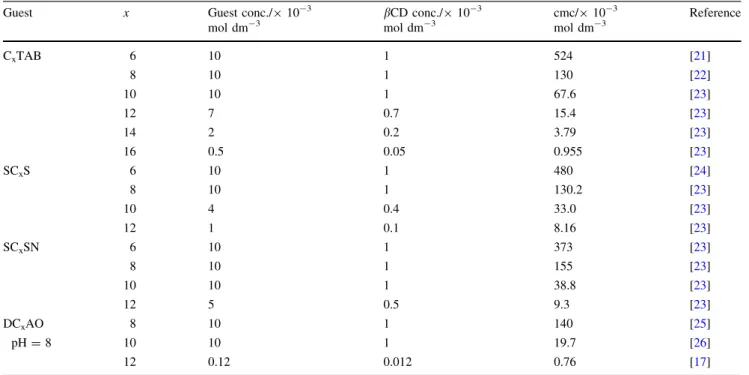
Table 1 Concentrations of surfactants (guests) andbCD (host) in aqueous solutions before thermometric titrations
Guest x Guest conc./910-3
mol dm-3
bCD conc./910-3 mol dm-3
cmc/910-3 mol dm-3
Reference
CxTAB 6 10 1 524 [21]
8 10 1 130 [22]
10 10 1 67.6 [23]
12 7 0.7 15.4 [23]
14 2 0.2 3.79 [23]
16 0.5 0.05 0.955 [23]
SCxS 6 10 1 480 [24]
8 10 1 130.2 [23]
10 4 0.4 33.0 [23]
12 1 0.1 8.16 [23]
SCxSN 6 10 1 373 [23]
8 10 1 155 [23]
10 10 1 38.8 [23]
12 5 0.5 9.3 [23]
DCxAO 8 10 1 140 [25]
pH=8 10 10 1 19.7 [26]
12 0.12 0.012 0.76 [17]
For comparison, the cmc values of the surfactants at 298 K are indicated
The area below each calorimeter peak i yielded a single point in the S-shaped reaction enthalpy curve (Fig.2):
DHi¼ Z
Pið Þdtt ð4Þ
Step-by-step summation of the peak areas provided the S-shaped enthalpy curve (Fig.2).
The analysis of the S-shaped curves by the Microcal OriginÒ 7 software is based on a one-set-of-site model fitting (VP-ITC tutorial guide) which provides the stoi- chiometry (N) of the complexation reaction, the binding constant (K), and the enthalpy of binding (DH).
Further, Eqs. (5) and (6) allow the calculation of the Gibbs free energy and the entropy term of the reaction [16]:
DG¼ RTlnK ð5Þ
TDS¼DHDG ð6Þ
Results and discussion
The thermodynamic parameters are listed in Table2. On average, the stoichiometry of the complexation was bCD:S=1:1. However, there was a critical chain length below which no complex formation was experienced:xB6 for the ionic surfactants andxB8 for the nonionic surfac- tants. This suggests that the nature of the headgroup influ- ences the complexation as the length of the alkyl chain decreases and hence the surfactant headgroup approaches the rim of thebCD ring. For the explanation, two phenomena should be considered: (i) the steric effect due to the different sizes of the headgroups; and (ii) possible interactions between the hydroxyl groups on the rim and the headgroup.
Figures3, 4, 5, and 6 depict the S-shaped enthalpy curves of the four homolog series studied. The complexa- tion became increasingly exothermic as the number of
carbon atoms (x) in the alkyl chain increased. The slope in the mid-section of the curves (at the inflection point) was proportional to the binding constantK. This is a measure of the stability of the complex: the larger the slope, the more stable the complex. K is plotted against the number of carbon atoms in Fig.7. It can be seen that, above the critical chain length, the effect of the headgroup on the stability of the complex was not significant. In contrast, the length of the alkyl chain exerted a dramatic effect onK:
its value increased nearly exponentially with increasing x.
The thermodynamic potential functions of the complex- ation reactions of the members of the four series of homologs are given in Table2 and a representative example is dis- played Fig. 8. DH, DG, and TDS varied linearly with the length of the alkyl chain. The magnitudes of these thermo- dynamic quantities were very similar for the three ionic surfactants, and even for the nonionic surfactants only dif- ferences of a few kJ mol-1were observed. Since the com- plexation was a spontaneous process,DGwas negative. The complexation reactions were favored by both the enthalpy and the entropy terms, irrespective of the length of the alkyl chain.
Before a discussion of the thermodynamics of the com- plexation ofbCD with surfactants at the molecular level, two important points should be noted. The cavity in a bCD molecule contains about 9 high energy water molecules [4].
Further, the hydrogen-bond density of the water around the surfactant molecules is higher than that of bulk water [29].
The complexation reaction can be divided into four con- secutive steps. Let us suppose that the guest is approaching the host. (i) Before it can enter the cavity in thebCD, the excess hydrogen-bond density around the surfactant mole- cule will disappear and the average hydrogen-bond density of hydrating water will become that of pure water, or water in solution distant from the dissolved surfactant molecule
0 2000 4000 6000 8000
28 29 30 31 32 33 34
dQ/dt/ μcal s–1
T /s
Fig. 1 Typical, exothermic calorimeter signal of a titration experi- ment. Titration of 1910-3mol dm-3 bCD with 10910-3 mol dm-3SC10S at 298 K
0.0 0.5 1.0 1.5 2.0 2.5
–9 –8 –7 –6 –5 –4 –3 –2 –1 0
ΔH/kJ mol–1
Molar ratio
Fig. 2 S-shaped enthalpy curve of the complexation reaction betweenbCD and SC10S at 298 K
(iceberg collapse [29,30]). This process is endothermic and accompanied by considerable entropy production. The ice- berg collapse plays the predominant role in the hydrophobic effect [29–31], which in turn makes a great contribution to the overall complexation of CDs with hydrophobic moieties [32,33]. (ii) Before the surfactant molecule can enter the cavity in thebCD, the high-energy water molecules must leave the interior of thebCD [32–34]. This process is exo- thermic and is accompanied by some entropy loss due to hydrogen bonding in the bulk solution. (iii) As the alkyl chain of the surfactant enters the hydrophobic cavity in the
bCD, heat is evolved due to favorable dispersion interac- tions. The magnitude of the entropy change of this process is presumably small. (iv) For short chain surfactants, interac- tion of the headgroup with the rim of thebCD may be con- sidered. Since both are hydrophilic, this interaction is supposed to be exothermic accompanied by some entropy loss. The four steps cannot be separated from one another;
only the enthalpy change of the resultant of the subprocesses can be detected calorimetrically. The knowledge of the thermodynamic parameters of inclusion complex formation at the phenomenological level may encourage computer Table 2 Thermodynamic parameters for the formation ofbCD-S inclusion complexes
Guest x N ± K/dm3mol-1 ± DH/kJ mol-1 ± DG/kJ mol-1 TDS/kJ mol-1
CxTAB 6 ø ø ø ø ø ø ø ø
8 1.24 0.091 650 80.2 -2.27 0.26 -16.06 13.78
10 0.96 0.004 4,990 80.3 -6.22 0.04 -21.11 14.89
12 1.09 0.004 16,700 450 -9.82 0.05 -24.10 14.29
14 1.02 0.002 46,300 692 -11.51 0.04 -26.63 15.12
16 1.07 0.021 64,000 868 -14.75 0.45 -27.43 12.68
SCxS 6 1.23 0.139 470 96 -5.68 0.94 -15.25 9.57
8 1.10 0.003 2,600 31 -7.05 0.03 -19.49 12.45
10 1.14 0.006 7,860 269 -9.69 0.07 -22.23 12.55
12 0.98 0.003 19,600 373 -12.47 0.06 -24.50 12.03
SCxSN 6 ø ø ø ø ø ø ø ø
8 1.28 0.031 693 33.5 -4.66 0.19 -16.21 11.55
10 1.22 0.010 5,860 298 -6.78 0.09 -21.51 14.73
12 1.04 0.004 16,700 441 -10.25 0.06 -24.10 13.86
DCxAO 8 ø ø ø ø ø ø ø ø
10 1.06 0.007 5,970 214 -5.10 0.05 -21.55 16.45
12 1.05 0.024 20,300 169 -6.39 0.23 -24.59 18.19
The data in the columns headed by ‘‘±’’ are the uncertainties. Symbol ‘‘ø’’ denotes when no complex formation was experienced
0.0 0.5 1.0 1.5 2.0 2.5
–11 –10 –9 –8 –7 –6 –5 –4 –3 –2 –1 0
H/kJ mol–1
Molar ratio
C8TAB C10TAB C12TAB C14TAB C16TAB
Δ
Fig. 3 Step-wise enthalpy change as a function of molar ratio [bCD]/
[CxTAB] at 298 K
0.0 0.5 1.0 1.5 2.0 2.5
–12 –11 –10 –9 –8 –7 –6 –5 –4 –3 –2 –1 0
H/kJ mol–1
Molar ratio
SC6S SC8S SC10S SC12S
Δ
Fig. 4 Step-wise enthalpy change as a function of molar ratio [bCD]/
[SCxS] at 298 K
scientists to simulate the complexation process at the molecular level.
Figure8 and Table2 demonstrate that, for each of the systems studied, the complexation reaction was exother- mic. This implies that the release of high-energy water from thebCD cavity and the dispersion interactions of the hydrophobic wall of the cavity with the surfactant tail overcompensated the iceberg collapse in the solution.
WhileDH became ever more negative with increasingx, TDSwas positive and, interestingly, displayed little varia- tion with change in x. This implies that the degree of freedom in the systems studied increases similarly.
DG markedly decreased asxincreased which is expected from the strong dependence ofK on x. Again, it may be concluded that the thermodynamics of the complexation depended more significantly on the length of the surfactant tail than on the nature of the surfactant head.
Our results can be compared with those reported from other laboratories. The complexation ofbCD with CxTABs (x=10, 12, 14) was earlier investigated by conducto- metric titration at four temperatures in the range 298–313 K [35]. With regards to the values ofKandDG, the agreement between the present calorimetric data and the conductometric results is satisfactory. However, the DH data calculated from the temperature dependence of DG conflict with the current, directly measured enthalpy data, e.g.,DH= -74.85 kJ mol-1(conductometry) versus DH= -6.22 kJ.mol-1 (calorimetry) for C10TAB at 298 K. Further, while conductometry predicts that the complexation should become less exothermic and be accompanied by entropy loss as x increases, our results reveal just the opposite trend. Further point of disagree- ment between the two sets of results is the fact that the stoichiometry of the complexation of C14TAB was found to be bCD:S=2:1, while in the present case N=1.
0.0 0.5 1.0 1.5 2.0 2.5
–10 –9 –8 –7 –6 –5 –4 –3 –2 –1 0
H/kJ mol–1
Molar ratio
SC8SN SC10SN SC12SN
Δ
Fig. 5 Step-wise enthalpy change as a function of molar ratio [bCD]/
[SCxSN] at 298 K
0.0 0.5 1.0 1.5 2.0 2.5
–5.0 –4.5 –4.0 –3.5 –3.0 –2.5 –2.0 –1.5 –1.0 –0.5
H/kJ mol–1
Molar ratio
DC10AO DC12AO
Δ
Fig. 6 Step-wise enthalpy change as a function of molar ratio [bCD]/
[DCxAO] at 298 K
6 8 10 12 14 16
0 10000 20000 30000 40000 50000 60000 70000
K/dm3 mol–1
Number of carbon atoms, x CxTAB
SCxS SCxSN DCxAO
Fig. 7 Binding constants K of the various bCD-S systems plotted against the number of carbon atomsxin the alkyl chain
8 10 12 14 16
–30 –25 –20 –15 –10 –5 0 5 10 15
H; G; TS/kJ mol–1
Number of carbon atoms, x
ΔH (CxTAB) ΔG (CxTAB) TΔS (CxTAB)
ΔΔΔ
Fig. 8 Thermodynamic potentials (DH, DG, and TDS) of the complexation reactions ofbCD with CxTABs at 298 K
Conductometric titration of bCD with alkyltrimethylam- monium chlorides, C12TACl and C14TACl [18] afforded Kvalues 2 orders of magnitudes lower than in the present work for C12TAB and C14TAB. For C12TACl and C14TACl,K/dm3mol-1values of 13,270 and 13,390 were determined in a static fluorescence study [18]. In the present work, K/dm3mol-1 increased from 16,700 (C12TAB) to 46,300 (C14TAB). Such a large difference cannot be attributed to the difference between the two counter ions, Cl-and Br-. We conclude that calorimetry is more suitable for the study of binding/complexation pro- cesses than most, if not all, experimental techniques. Our results can further be compared with those of the com- plexation of bCD with (3-alkoxy-2-hydroxypropyl)trime- thylammonium bromides, CxHpTAB (x=8, 10, 12), studied with a heat-flow titration microcalorimeter [19].
Although the thermodynamic potentials were comparable both in magnitude and trend to those for the CxTABs, the changes inK,DH,DG, andTDS were found to be signif- icantly larger for CxHpTAB [19]. We conclude that titra- tion microcalorimetry yields more reliable thermodynamic data than any other indirect method based on the temper- ature dependence of the particular experimental quantity measured.
Conclusions
Isothermal titration microcalorimetry indicated that bCD forms 1:1 complexes with the even-carbon-number mem- bers of the four series of surfactant homologs: CxTABs, SCxSs, SCxSNs, and DCxAOs at 298 K. However, there was a critical chain length below which no complexation occurred. The effect of the nature of the headgroup on the complexation was small. In contrast, the stability of the complex was dramatically influenced by the number of carbon atoms (x) in the alkyl chains of the surfactants. The binding constantKincreased practically exponentially with increasingx. The thermodynamic parametersDH,DG,and TDS proved to be linear functions of x. DGwas negative and the complexation reactions ware favored by both the enthalpy and the entropy terms. In each case, the com- plexation reaction was exothermic and DH became ever more negative with increasing x. TDS was positive, but demonstrated little variation with change inx. Calorimetry is a more powerful method for study of the thermody- namics of complexation than any currently applied other experimental method.
Acknowledgements This work was supported by the Hungarian Science Foundation (OTKA 68152), TA´ MOP-4.2.1/B-09/1/KONV- 2010-0005—Creating the Center of Excellence at the University of Szeged, and the European Union, and co-financed by the European Regional Development Fund.
References
1. Szejtli J. Cyclodextrin technology. Dordrecht: Kluwer; 1988.
2. Szejtli J. Cyclodextrins and their inclusion complexes. Budapest:
Academic Press; 1982.
3. Del Valle EMM. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–46.
4. Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98:1743–54.
5. Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11.
6. Rowe RC, Sheskey PJ, Quinn ME, editors. Handbook of phar- maceutical excipients. 6th ed. London: RPS Publishing; 2009.
p. 210–4.
7. Wszelaka-Rylik M, Gierycz P. Isothermal titration calorimetry (ITC) study of natural cyclodextrins inclusion complexes with drugs. J Therm Anal Calorim. 2012. doi 10.1007/s10973-012- 2251-4.
8. Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical sol- ubilizers. Adv Drug Deliv Rev. 2007;59:645–66.
9. Singh M, Sharma R, Banerjee UC. Biotechnological applications of cyclodextrins. Biotechnol Adv. 2002;20:341–59.
10. Szente L, Szejtli J. Cyclodextrins as food ingredients. Trends Food Sci Technol. 2004;15:137–42.
11. Crini G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci.
2005;30:38–70.
12. Rosen MJ. Surfactants and interfacial phenomena. 2nd ed. New York: Wiley; 1989.
13. Myers D. Surfactant science and technology. New York: VCH;
1988.
14. Blokzijl W, Engberts JBFN. Hydrophobic effects: opinion and facts. Angew Chem Int Ed. 1993;32:1545–79.
15. Bai Y, Xu G-Y, Xin X, Sun H-Y, Zhang H-X, Hao A-Y, Yang X-D, Yao L. Interaction between cetyltrimethylammonium bro- mide and b-cyclodextrin: surface tension and interfacial dila- tional viscoelasticity studies. Colloid Polym Sci. 2008;286:
1475–84.
16. Benk}o M, Kira´ly Z. Thermodynamics of inclusion complex for- mation of b-cyclodextrin with a variety of surfactants differing in the nature of headgroup. J Chem Thermodyn. 2012;54:211–6.
17. Tuncay M, Christian SD. A study of the binding of dimethyld- odecylamine oxide by b-cyclodextrin using surface tension measurements. J Colloid Interface Sci. 1994;167:181–5.
18. Sun D-Z, Wang S-B, Wei X-L, Yin B-L. A microcalometric study of b-cyclodextrin with 3-alkoxyl-2-hydroxypropyl tri- methyl ammonium bromides in aqueous solution. J Chem Ther- modyn. 2005;37:431–6.
19. Mehta SK, Bhasin KK, Dham S, Singla ML. Micellar behavior of aqueous solutions of dodecyldimethylethylammonium bromide, dodecyltrimethylammonium chloride and tetradecyltrimethylammonium chloride int he presence ofa-,b-, HPb- andc-cyclodextrins. J Colloid Interface Sci. 2008;321:442–51.
20. Cooper A. Microcalorimetry of heat capacity and volumetric changes in biomolecular interactions-the link to salvation?
J Therm Anal Calorim. 2011;104:69–73.
21. Mosquera V, del Rı´o JM, Attwood D, Malcolm MG, Jones N, Prieto G, Suarez MJ, Sarmiento F. A study of the aggregation behavior of hexyltrimethylammonium bromide in aqueous solu- tion. J Colloid Interface Sci. 1998;206:66–76.
22. Tadros TF. Applied surfactants: principles and applications.
Weinheim: Wiley; 2005.
23. van Os NM, Haak JR, Rupert LAM. Physico-chemical properties of selected anionic, cationic and nonionic surfactant. Amsterdam:
Elsevier; 1993.
24. Sua´rez MJ, Lo´pez-Fonta´n JL, Sarmiento F, Mosquera V. Study of the aggregation behavior of sodium n-hexyl sulfate in aqueous solution. Langmuir. 1999;15:5265–70.
25. Kresheck GC. The temperature dependence of the heat capacity change for micellization on nonionic surfactants. J Colloid Interface Sci. 2006;298:432–40.
26. Kira´ly Z, Findenegg GH. Calorimetric study of the adsorption of short-chain nonionic surfactants on silica glass and graphite:
dimethyldecylamine oxide and octyl monoglucoside. Langmuir.
2000;16:8842–9.
27. Turnbull WB, Daranas AH. On the value of c: can low affinity systems by studied by isothermal titration calorimetry. J Am Chem Soc. 2003;125:14859–66.
28. Wisemann T, Williston S, Brandts J, Lin L. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989;179:131–7.
29. Ne´methy G, Scheraga HA. Structure of water and hydrophobic bonding in proteins. I. A model for the thermodynamic properties of liquid water. J Chem Phys. 1962;36:3382–400.
30. Frank HS, Evans MW. Free volume and entropy in condensed systems III. Entropy in binary liquid mixtures; partial molal entropy in dilute solutions; structure and thermodynamics in aqueous electrolytes. J Chem Phys. 1945;13:507–33.
31. Tanford C. The hydrophobic effect: formation of micelles and biological membranes. New York: Wiley; 1973.
32. Liu L, Guo Q-X. The driving forces in the inclusion complexa- tion of cyclodextrins. J Incl Phenom Macrocycl Chem. 2002;42:
1–14.
33. Steed JW, Atwood JL. Supramolecular chemistry. London:
Wiley; 2000. p. 19–30.
34. Dodziuk H. Molecules with holes—cyclodextrins. In: Dodziuk H, editor. Cyclodextrins and their complexes: chemistry, analytical methods, applications. Weinheim: Wiley; 2006. p. 1–30.
35. Rafati AA, Bagheri A, Iloukhani H, Zarinehzad M. Study of inclusion complex formation between a homologous series of n-alkyltrimethylammonium bromides and b-cyclodextrin, using conductometric technique. J Mol Liq. 2005;116:37–41.
![Fig. 3 Step-wise enthalpy change as a function of molar ratio [bCD]/](https://thumb-eu.123doks.com/thumbv2/9dokorg/1190774.87847/5.892.78.814.106.468/fig-step-wise-enthalpy-change-function-molar-ratio.webp)
![Fig. 5 Step-wise enthalpy change as a function of molar ratio [bCD]/](https://thumb-eu.123doks.com/thumbv2/9dokorg/1190774.87847/6.892.83.434.89.347/fig-step-wise-enthalpy-change-function-molar-ratio.webp)