UNIVERSITY OF PANNONIA
DOCTORAL SCHOOL OF MOLECULAR AND NANOTECHNOLOGIES
IMMOBILIZATION OF β- D -GALACTOSIDASE ON NANOSTRUCTURED CARRIERS AND
CHARACTERIZATION OF THE OBTAINED BIOCATALYSTS
Ph.D. THESIS
AUTHOR:
BIRÓ EMESE
CHEMICAL ENGINEER
SUPERVISORS:
GYENIS JÁNOS DSc
PROFESSOR EMERITUS
SISAK CSABA CSc
ASSOCIATE PROFESSOR
UNIVERSITY OF PANNONIA
RESEARCH INSTITUTE OF CHEMICAL AND PROCESS ENGINEERING 2012
Értekezés doktori (PhD) fokozat elnyerése érdekében
Írta: Biró Emese, okleveles vegyészmérnök
Készült a Pannon Egyetem Molekuláris- és Nanotechnológiák Doktori Iskolája keretében
Témavezetők: DR. Gyenis János, professor emeritus Dr. Sisak Csaba, egyetemi docens
Elfogadásra javaslom (igen / nem)
……….
(aláírás) A jelölt a doktori szigorlaton ...%-ot ért el,
Az értekezést bírálóként elfogadásra javaslom:
Bíráló neve: …... …... igen /nem
……….
(aláírás) Bíráló neve: …... …...) igen /nem
……….
(aláírás) Bíráló neve: …... …...) igen /nem
……….
(aláírás) A jelölt az értekezés nyilvános vitáján …...%-ot ért el.
Veszprém, ……….
a Bíráló Bizottság elnöke A doktori (PhD) oklevél minősítése…...
………
Az EDHT elnöke
TABLE OF CONTENTS
Page
TABLE OF CONTENTS i
LIST OF ABBREVIATIONS AND NOTATIONS iv
ABSTRACT v
KIVONAT vi
AUSZUG vii
INTRODUCTION. AIM OF THE THESIS viii
1. LITERATURE SURVEY 1
1.1. Overview of enzyme immobilization 1
1.2. Methods of enzyme immobilization 4
1.2.1. Physical methods 5
1.2.2. Chemical methods 7
1.3. Supports for enzyme immobilization 8
1.3.1. Types of support materials 9
1.3.1.1. Inorganic carriers 10
1.3.1.2. Organic carriers 18
1.3.2. Particulate forms of supports applied in biocatalysis 28
1.3.2.1. Composite particles 32
1.3.2.2. Microspheres and nanospheres 36
1.3.1.3. Chitosan as support for enzyme immobilization 39
1.3.1.4. Sol-gel matrices as immobilization carriers 42
1.4. Immobilization of β-D-galactosidase 44
1.4.1. Structure and reaction mechanism of β-D-galactosidase 46
1.4.2. Sources of β-D-galactosidase 48
1.4.3. Methods for immobilization of β-D-galactosidase 50
1.4.3.1. Adsorption 51
1.4.3.2. Covalent binding 52
1.4.3.3. Entrapment 54
1.4.4. Applications of β-D-galactosidase 55
2. MATERIALS AND METHODS 58
2.1. Raw materials 58
2.2. Preparation of chitosan macro-, micro-, and nanoparticles 59
2.2.1. Preparation of chitosan macrospheres 59
2.2.2. Preparation of chitosan microparticles 59
2.2.2.1. Chitosan microparticles by precipitation with spraying 60 2.2.2.2. Chitosan microparticles by ionotropic gelation 60 2.2.2.3. Chitosan microparticles by emulsion cross-linking 60
2.2.3. Preparation of chitosan nanoparticles 61
2.3. Experimental procedure of three-step experimental design 61
2.4. Analytical methods 62
2.4.1. Enzyme activity assay 62
2.4.2. Bradford method for protein content determination 64 2.4.3. Determination of D-glucose by glucose oxidase-peroxidase method 65
2.4.4. Particle size measurement by laser diffraction 67
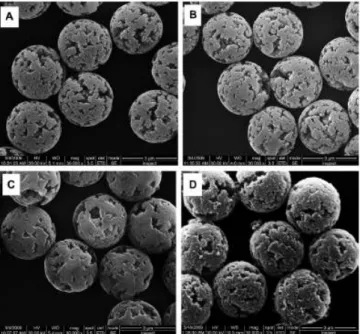
2.4.5. Scanning electron microscopy (SEM) 68
2.4.6. Transmission electron microscopy (TEM) 68
2.5. Experimental protocols of β-D-galactosidase immobilization 68 2.5.1. β-D-galactosidase immobilization onto chitosan macro-, micro-, and
nanoparticles by covalent binding 68
2.5.2. β-D-galactosidase immobilization by sol-gel entrapment 69 2.5.3. β-D-galactosidase immobilization by sol-gel entrapment combined with
adsorption 69
2.5.4 Protein labeling with fluorescein isothiocyanate (FITC) 70 2.6. Kinetic parameters of o-nitrophenyl-β-D-galactopyranoside and lactose
hydrolysis by native and immobilized β-D-galactosidases 71 2.7. Time course of lactose hydrolysis by native and immobilized
β-D-galactosidases 72
2.8. Reuse of biocatalysts 72
3. RESULTS AND DISCUSSION 73
3.1. Preparation of chitosan support materials for enzyme immobilization 74
3.1.1. Chitosan macrospheres by precipitation method 74
3.1.2. Chitosan microparticles 76
3.1.2.1. Preparation methods of chitosan microparticles 76 3.1.2.2. Influence of experimental parameters on the formation of chitosan
microspheres by the emulsion cross-linking method 78 3.1.3. Chitosan nanoparticles obtained by ionotropic gelation 84 3.2. Experimental design to determine the effect of process parameters on
the size of chitosan microspheres obtained by emulsion cross-linking
method 87
3.2.1. Effect of stirring rate 96
3.2.2. Effect of surfactant concentration 98
3.2.3. Effect of chitosan concentration 99
3.2.4. Effect of glutaraldehyde 101
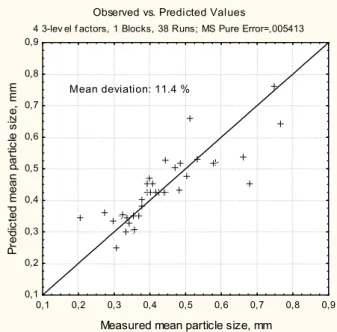
3.2.5. Prediction of the mean particle size 102
3.2.6. Possibilities to control the mean particle size 104 3.3. Covalent immobilization of β-D-galactosidase on chitosan supports 107 3.3.1. Evaluation of different chitosan-based carriers for covalent binding of
β-D-galactosidase 107
3.3.2. Optimization of the immobilization parameters on chitosan
microparticles 110
3.3.2.1. Influence of the loaded protein concentration 111
3.3.2.2. Influence of glutaraldehyde concentration 113
3.3.2.3. Influence of immobilization time 115
3.3.2.4. Improvement of the catalytic efficiency by reduction with sodium
borohydride 116
3.4. Sol-gel entrapment of β-D-galactosidase in organic-inorganic composite matrices obtained from alkyl-trialkoxy and tetra-alkoxy silane
precursors 117
3.4.1. Influence of nature of alkyl group of silane precursors on the activity of immobilized β-D-galactosidase
117 3.4.2. Influence of the experimental setup of sol-gel matrix on the activity of
immobilized β-D-galactosidase 120
3.4.3. Influence of molar ratio of silane precursors on the activity of sol-gel
immobilized β-D-galactosidase 121
3.4.4. Influence of nature of additives on the activity of immobilized
β-D-galactosidase 122
3.4.5. Influence of enzyme loading on the activity of immobilized
β-D-galactosidase 124
3.4.6. Reproducibility of the immobilization method 125
3.5. Immobilization of β-D-galactosidase in organic-inorganic composites
obtained by sol-gel entrapment combined with adsorption 126 3.6. Characterization and stability of the immobilized enzymes 131 3.6.1. pH profile of native and immobilized β-D-galactosidases 131 3.6.2. Thermostability of native and immobilized β-D-galactosidases 132 3.6.3. Protein labeling with FITC of immobilized β-D-galactosidases 134 3.6.4. Study of kinetic parameters of native and immobilized
β-D-galactosidases on synthetic (o-nitrophenyl-β-D-galactopyranoside)
and natural (lactose) substrates 136
3.6.5. Time course of lactose hydrolysis by native and immobilized
β-D-galactosidases 141
3.6.6. Multiple utilization of immobilized β-D-galactosidases in batch
hydrolysis of o-nitrophenyl-β-D-galactopyranoside and lactose 142
3.6.7. Storage stability of the immobilized enzymes 143
4. SUMMARY 145
5. NEW SCIENTIFIC RESULTS/ THESES 148
6. ÚJ TUDOMÁNYOS EREDMÉNYEK/ TÉZISPONTOK 152
7. REFERENCES 157
LIST OF PUBLICATIONS 173
CITATIONS 175
KÖSZÖNETNYILVÁNÍTÁS 178
LIST OF ABBREVIATIONS AND NOTATIONS
4AAP 4-aminoantipyrine A.U. absorbance units BSA bovine serum albumin
d mean particle size
DMeDMOS dimethyldimethoxysilane
DMF dimethylformamide
ESEM environmental scanning electron microscope EtTEOS ethyltriethoxysilane
EtTMOS ethyltrimethoxysilane
ε relative mean deviation between the measured and predicted mean particle size
FITC fluorescein isothiocyanate GOD glucose oxidase
HLB hydrophilic-lipophilic balance iBuTEOS isobutyltriethoxysilane
iBuTMOS isobutyl trimethoxysilane iPrOH isopropyl alcohol
K number of the independent variables (factors) kcat catalytic constant
kcat/ Km catalytic efficiency of the enzymes expressed by the kinetic constants kH hydrolytic constant
Km apparent Michaelis-Menten constant
L number of levels (different values) ofthe independent variables MeTEOS methyltriethoxysilane
MeTMOS methyltrimethoxysilane
N number of the necessary experiments
n stirring rate
NMWL nominal molecular weight limit ONP o-nitrophenol
ONPG o-nitrophenyl-β-D-galactopyranoside PEG 20000 polyethylene glycol 20000
PEG 4000 polyethylene glycol 4000
POD peroxidase
PrTMOS propyltrimethoxysilane PVA polyvinyl alcohol
SEM scanning electron microscopy TEM transmission electron microscopy TEOS tetraethoxysilane
TMOS tetramethoxysilane TPP sodium tripolyphosphate Vmax maximal initial rate xCh chitosan concentration xGl glutaraldehyde concentration xTw Tween 80 surfactant concentration
β-D-gal β-D-galactosidase from Kluyveromyces lactis
ABSTRACT
IMMOBILIZATION OF β-D-GALACTOSIDASE ON NANOSTRUCTURED CARRIERS AND CHARACTERIZATION OF THE OBTAINED BIOCATALYSTS
Immobilization of β-D-galactosidase represents an important approach for development of lactose hydrolysis technology. Chitosan proved to be an excellent carrier for β-D- galactosidase immobilization, by both covalent binding and sol-gel entrapment combined with adsorption.
Among different preparation methods of chitosan support particles for covalent binding, emulsion cross-linking was the most efficient, producing narrow-sized microspheres, without aggregation. Influences of the most significant process variables: stirring rate, concentrations of chitosan, glutaraldehyde cross-linker, and Tween 80 emulsifier, were studied by experimental design and statistical analysis. Concentration of chitosan was the critical parameter, but it must be correlated with other process variables in order to obtain particles with desired mean size.
The best process parameters of β-D-galactosidase immobilization by covalent coupling on chitosan microspheres were found as 3% glutaraldehyde cross-linker concentration, enzyme loading of 27 mg protein/g dry support, 6 h reaction time, and reduction of the formed covalent double bonds to more flexible secondary amino bonds. In the best conditions, 23.5% of the total enzymatic activity was recovered after immobilization.
Much higher total enzymatic activity recovery yields, up to 400%, were obtained using the sol-gel immobilization method, demonstrating that encapsulation resulted in activation of the enzyme. Tetraethoxysilane and methyltriethoxysilane precursors, at 7:1 molar ratio and protein loading of 4.1 mg/mmol silane were the immobilization parameters leading to the highest biocatalytic efficiency.
Sol-gel entrapment combined with adsorption on chitosan yielded activity as high as obtained for simple sol-gel entrapment.
Beside better pH and thermal stability, the immobilized β-D-galactosidases demonstrated high storage stability and availability for multiple uses, as the sol-gel entrapped enzyme maintained 50% of the initial activity after 5 reaction cycles.
KIVONAT
β-D-GALAKTOZIDÁZ IMMOBILIZÁLÁSA NANOSZERKEZETŰ
HORDOZÓKRA ÉS AZ ELŐÁLLÍTOTT BIOKATALIZÁTOROK VIZSGÁLATA
A β-D-galaktozidáz immobilizálása fontos módszer a laktóz hidrolízis technológia tökéletesítésében. A kitozán kiváló tulajdonságokkal rendelkező hordozónak bizonyult β-
D-galaktozidáz immobilizálására, mind a kovalens kötéssel történő rögzítés, mind az adszorpcióval kombinált szol-géles bezárás esetében.
A β-D-galaktozidáz rögzítésére alkalmas kitozán részecskék előállítási módszerei közül az emulziós keresztkötés bizonyult a leghatékonyabbnak, mivel szűk méreteloszlású egyedi részecskéket eredményezett. A legszignifikánsabb technológiai paraméterek, így a keverési sebesség, a kitozán, a glutáraldehid keresztkötő ágens és a Tween 80 emulgeálószer koncentrációk hatásainak vizsgálata kísérlettervezési módszer alkalmazása és a kísérleti eredmények statisztikai kiértékelése révén történt. A kitozán oldat koncentrációja kulcsfontosságú paraméternek bizonyult, de ez szoros kölcsönhatásban állt a többi technológiai paraméter értékeivel is, melyeket mind figyelembe kell venni annak érdekében, hogy a kívánt átlagméretű részecskék előállítása váljon lehetségessé.
A β-D-galaktozidáz kovalens kötéssel történő rögzítésénél a következő technológiai paraméterek eredményezték a legjobb kötésre vitt aktivitás visszanyerési értékeket: 3%-os glutáraldehid koncentráció, 27 mg fehérje/(g száraz hordozó) kötésre vitt enzim tartalom, és 6 óra reakció idő az enzim kapcsolásához. Ugyancsak szükségesnek bizonyult a keletkezett dupla kovalens kötések rugalmasabb szekunder amino kötéseket eredményező redukciója. A legjobb rögzítési körülmények között a kötésre vitt enzimatikus aktivitás 23,5%-a nyerhető vissza.
A szol-géles rögzítési módszerrel sokkal magasabb, 400% feletti kötésre vitt aktivitást lehett visszanyerni, ami arra mutat, hogy ez a rögzítési módszer elősegítette az enzim aktiválódását. A legmagasabb biokatalitikus hatásfokhoz a tetraetoxiszilán és metiltrietoxiszilán prekurzorok 7:1 mólaránya és 4,1 mg fehérje/mmol szilán kötésre vitt enzim tartalom vezettek. Szol-géles bezárás és adszorpció kombinálása (kitozánt használva adszorbensként) hasonló aktivitást eredményezett, mint a szimpla szol-gél bezárás módszere.
A rögzített β-D-galaktozidázok jobb pH és termostabilitásuk mellett nagyobb tárolási stabilitást és többszörös felhasználhatósági lehetőséget mutattak: így például a szol-géles bezárással rögzített enzim öt reakció ciklus után megőrizte kezdeti aktivitásának 50%-át.
AUSZUG
IMMOBILISIERUNG VON β-D-GALACTOSIDASE AUF
NANOSTRUKTURIERTEN TRÄGERN UND CHARAKTERISIERUNG DER ERHALTENEN BIOKATALYSATOREN
Immobilisierung von β-D-Galactosidase vertritt einen Ansatz für die Verbesserung von Lactose-Hydrolysetechnologie. Chitosan erwies sich zu einem ausgezeigneten Trӓger für die Immobilisirung von β-D-Galactosidase, sowohl für die kovalente Bindung als auch für das Sol-Gel-Einschliessen kombiniert mit Adsorption.
Unten den verschiedenen Verarbeitungsverfahren von den Chitosan-Partikeln, die für die Immobilisirung von β-D-Galactosidase geignet waren, war die Emulsionsvernetzung das effizienteste, wobei sehr kleine Mikrokügelchen ohne Aggregation gebildet wurden. Die Wirkung der wichtigsten Prozessvariablen: Rührgeschwindigkeit, Konzentrationen von Chitosan, Glutaraldehyd als Vernetzer und Emulgator Tween 80 waren mit Hilfe von experimentellen Versuchsplanung und statistischen Analyse studiert. Die Konzentration von Chitosan war der kritische Parameter, aber er musste mit den anderen Prozessvariablen korreliert werden, um Teilchen mit der gewünschten Größe zu bekommen.
Die besten Prozessvariablen der β-D-Galactosidase-Immobilisierung mit kovalenter Bindung auf Chitosan-Mikrosphären sind 3% Konzentration von Glutaraldehyd Vernetzer, Enzymladung von 27 mg Protein/g trockener Trӓger, 6 h Reaktionszeit und Reduzierung der kovalenten Doppelbindungen auf die flexibleren sekundären Aminobindungen. Bei den besten Parametern 23,5% der gesamten enzymatischen Aktivitӓt wurde nach der Immobilisierung zurückgewonnen.
Viel höhere (bis auf 400%) gesamte enzymatische Aktivitӓt wurde mit der Sol-Gel- Immobilisierung erreicht, was demonstriert, dass das Enzym durch die Kapselung aktiviert wird. Die Vorläufer Tetraethoxysilan und Methyltriethoxysilan bei einer Molverhӓltnis von 7:1 und Proteinmenge von 4,1 mg/mmol Silan waren die Immobilisierungsparameter, die zu den höchsten biokatalytischen Wirkung führten.
Sol-Gel-Einschliessen kombiniert mit Adsorption auf Chitosan brachte ӓhnliche Aktivitӓt als einfaches Sol-Gel-Einschliessen.
Das immobilisierte β-D-Galactosidase zeigte neben besserem pH und thermischer Stabilität hohe Lagerstabilität und konnte in mehreren Reaktionen verwendet werden. Das Enzym mit Sol-Gel-Einschliessen behielt 50% seiner originellen Aktivität nach 5 Reaktionszyklen.
INTRODUCTION. AIM OF THE THESIS
In the past decades, biocatalysis has emerged as an increasingly attractive domain for both science and industry, with regard to the high specificity and selectivity and advantageous operation conditions compared to chemical catalysis. Nature provided a large scale of biological catalysts to operate most effectively under physiological conditions and on natural substrates. However, naturally occurring enzymes were usually not suitable for biocatalytic processes, without further tailoring or redesign of the enzyme itself, in order to fine-tune substrate specificity and activity. The spectacular development of biological engineering techniques produced enzymes that are more and more appropriate for preparation of complex, high value materials, but the inherent instability of biocatalysts still remains a problem to be resolved.
Immobilization could be a solution to improve enzyme stability, allowing recovery and reuse of these, mostly expensive, catalysts. Moreover, utilization of immobilized enzymes permits to greatly simplify the design of industrial reactors and to facilitate the control of process parameters. Therefore, immobilization of enzymes could be, in short time, a requirement of industrial utilization.
Several immobilization techniques have been developed in the past decades, some of them being industrially used from a long time. However, the diversity of enzymes and applications enforces the development of new methods or improvement of the existent ones. An appropriate design of immobilization procedures could also have beneficial effect on development of the whole biocatalysis domain.
The main aim of this thesis was to develop new immobilized biocatalysts for hydrolysis of lactose. Enzymatic hydrolysis of lactose is one of the most important biotechnological processes in the food industry. It is realized by β-D-galactosidase, or lactase, an enzyme used in several applications for hydrolysis of lactose from milk or whey. The main benefit of lactose-hydrolyzed products is that they represent one of the possible approaches to diminish lactose intolerance, present in more than half of population of the world. Another important application of β-D-galactosidase is formation of galacto-oligosaccharides, important probiotic compounds.
The experimental setup of this thesis was focused on two directions: (i) preparation of micro- and nanoscaled chitosan support materials for immobilization of β-D-galactosidase and investigation of parameters that could improve the efficiency of covalent
immobilization onto these carriers, and (ii) preparation of nanostructured biocatalysts by the sol-gel method, as a new possibility to obtain immobilized β-D-galactosidase with high catalytic efficiency and improved stability. The sol-gel technique offers amazing possibilities to fine-tune the parameters of immobilization protocol to the specific requirements of a given enzyme and application. Other important aim was to elaborate an experimental design which could allow elucidation of influence of the main process parameters on chitosan particles’ size. Such a model is important to reduce the number of experiments and facilitate finding of the optimal values.
Finally, the last task was characterization of the obtained biocatalysts and evaluation of their stability, as increase of stability is the main objective and advantage of enzyme immobilization.
1. LITERATURE SURVEY
1.1. Overview of enzyme immobilization
Enzymes are very specific and efficient catalytically active proteins, which can work without need of extreme temperatures, high pressures or corrosive conditions, as it is the case for the majority of chemical catalysts. Especially in the last decades, they became increasingly important in a wide range of industrial sectors, to replace the classical chemical catalysts. The reactions catalyzed by enzymes are mostly one-step processes, carried out with high efficiency, specificity and reaction rate. This scientific domain is usually referred to as “biocatalysis” and the processes are described as “bioconversions” or
“biotransformations”. The enzymes used in industrial biotechnology and biocatalysis are practically all obtained from microorganisms via fermentation. Industrial enzymes represent a two billion dollar sector in industrial biotechnology (Soetaert and Vandamme, 2006).
Nowadays, one of the most important driving forces in science is to develop green, sustainable technologies for manufacture of chemical compounds. Beyond the mild reaction conditions, shorter synthetic routes, biodegradability of the catalyst and use of environmentally friendly solvents, biocatalytic processes are also characterized by high chemo-, regio-, and stereoselectivities, resulting in lower amount of generated waste.
Despite their excellent catalytic properties, some characteristics of enzymes are not adequate for industrial applications: difficult recovery for reutilization, unsatisfactory thermal, pH, storage and operational stability, possible inhibition by substrates and products, low selectivity against non-natural substrates. Therefore, enzyme properties need to be significantly improved before their use as industrial catalysts. There are several tools to achieve such an improvement (Guisan, 2006):
• synthetic biology techniques;
• immobilization;
• reaction and reactor engineering.
For both technical and economic reasons, most enzyme-catalyzed processes require re-use or continuous use of the biocatalyst for an extended period of time. Under this perspective, immobilization of enzymes may be defined as any technique that is able to allow the reuse or continuous use of the biocatalysts.
The potential advantages associated with the immobilization of biocatalysts can be resumed as follows (End and Schöning, 2004):
• Higher stability with regard to temperature, pH, and catalyst poisoning;
• Repeated use of biocatalysts;
• Higher resistance to shear stress and contamination;
• Higher activity by better availability of catalytic centres (in certain cases);
• Ease of developing continuous processes;
• Easy separation from reaction media (easier downstream processing);
• Increased reaction rate due to high catalyst concentration (for certain reactor types).
The most important characteristics of immobilized enzymes are simplicity, cost effectiveness and stability (Guisan, 2006). It must be pointed out that improvement of enzyme stability can be targeted in several ways. Besides immobilization, protein engineering, chemical modification of enzymes, utilization of additives (Iyer and Ananthanarayan, 2008), reaction medium engineering, e.g. utilization of ionic liquids (Fehér et al., 2007), are all valuable methods to increase the stability of enzymes. The efficient recovery and reuse of costly enzymes is considered the main requirement for economic viability of a biocatalytic process. Immobilization enables use of the enzymes in continuous, fixed-bed operation. Enhanced stability, under both storage and operational conditions, and improved enzyme performance result in higher catalyst productivities and lower costs per kg product. Furthermore, immobilization leads to more convenient handling of the enzyme, minimizing or eliminating protein contamination of the product (Sheldon, 2007a).
Immobilized biocatalysts have been used for industrial production of chemical intermediates and products since the end of the 1960’s. The first industrial plant was operated in Japan, for optical resolution of D,L-amino acids with aminoacylase immobilized by ionic adsorption on DEAE-Sephadex (Chibata, 1978). Straathof et al.
analyzed 134 industrial biotransformation processes, and revealed that hydrolases (44%) and oxidoreductases (30%) were the most frequently used enzyme classes. The majority (89%) of the manufactured products were chiral, used as fine chemicals. Despite the generally recognized advantages of immobilized enzymes, only 20 of these 134 processes were realized with immobilized enzymes (Straathof et al., 2002). Despite a plausible improvement in the last ten years (data are not available) it is obvious that the number of
industrial processes employing immobilized enzymes remains still low. It is not easy to answer why the number of such processes is not significantly higher. Evidently, there are some disadvantages linked with the utilization of immobilization enzymes (End and Schöning, 2004):
• The necessity to set-up an immobilization process;
• Supplementary costs caused by support and additional reagents;
• Increased mass transfer resistances (diffusion limitations) in the reaction systems working with immobilized enzymes.
Immobilization can be a considerable cost factor. Several biotransformations can be more efficiently realized under fermentative conditions, with living or resting cells, eliminating the costs of immobilization. It is also well known that not only isolated enzymes may be immobilized, but also living or resting cells and such processes may be also cheaper.
Another cost-rising factor is lack of a universally applicable method of enzyme immobilization. There are two major tasks which have to be addressed during the build-up of an efficient immobilized enzyme technology (Cao, 2005a):
• Selection of a suitable carrier (defined as the non-catalytic part of an immobilized enzyme, on which the catalytic part is constructed), process parameters (pH, temperature, and nature of medium), and enzyme itself (source, nature and purity);
• Fulfilment of catalytic needs (productivity, space–time yield, stability and selectivity) and non-catalytic needs (separation, control, down-streaming process) for the given application;
As it was stated by Cao, the availability of a robust immobilized enzyme at an early stage of the biocatalytic process would save cost for both process development and large-scale production. Unfortunately, guidelines for selection of an appropriate immobilization method and prediction of the immobilized enzyme performance in a specific application are still missing; therefore implementation of a rational approach for immobilized enzymes design was not realized to date (Cao, 2005a). Nevertheless, enzyme immobilization continues to attract considerable attention from researchers in both industry and academia.
Novel concepts and methods continue to appear, but many of these innovations are unlikely to be applied at industrial scale, as they involve very difficult immobilization techniques or use supports which are substantially more expensive than the enzyme to be
immobilized (Sheldon, 2007a). However, such biocatalysts could be interesting for biosensors or other domains where the cost contribution of the enzyme is less important.
It is well known that, beyond the main utilization as heterogeneous biocatalysts in chemical, pharmaceutical and food industry, immobilized enzymes have several other applications. Some significant examples are:
• Sensors for biomedical applications and bioreactors for treatment of enzyme- deficient diseases (Liang et al., 2000);
• Sensors for analytical flow systems (Twyman, 2005);
• Enzymatically controlled drug delivery (Fischel-Ghodsian et al., 1988);
• Purification of proteins and enzymes by biospecific adsorption (Cao, 2005b).
The existent industrial biocatalytic processes were comprehensively reviewed by Liese et al. Major industrial applications of immobilized biocatalysts are related to production of sugars, amino acids, chemical and pharmaceutical products, as results from Table 1.1.
Table 1.1. Main products manufactured at industrial scale using immobilized enzymes (Liese et al., 2006)
Product Enzyme Capacity (tones/year)
High fructose corn syrup glucose isomerase (xylose isomerase)
> 7,000,000
Acrylamide nitrile hydratase* > 30,000
6-Aminopenicillanic acid penicillin acylase 2,000
L-amino acids aminoacylase > 300
*immobilized microbial cells are mainly employed
A number of immobilization carriers are commercially available, even for applications at industrial scale, like as Eupergit (oxirane acrylic beads, Röhm GmbH, Germany), Accurel (polypropylene powder, Membrana GmbH, Germany), as well as Celite or sodium alginate (End and Schöning, 2004).
1.2. Methods of enzyme immobilization
Up to date, a universally applicable method for immobilization of all enzymes or for all applications of a certain enzyme does not exist. The choice of a certain procedure, support, or enzyme strongly depends on the chosen process and must be evaluated from case to case. The development of an immobilized procedure can be laborious and expensive (End
and Schöning, 2004). Based on the nature of interaction that leads to immobilization, immobilization methods are classified as physical and chemical methods (Péter, 2005;
Seville, 2011). A different approach is to classify immobilization methods as irreversible and reversible (Guisán, 2006). According to another largely used classification system, three types of immobilization can be distinguished: (i) binding to a carrier, (ii) entrapment (encapsulation) and (iii) cross-linking (Chibata, 1978; Sheldon, 2007a).
In this section, the classification based on nature of interaction (physical or chemical) that leads to immobilization was adopted. As the subject of this thesis is immobilization of β-D- galactosidase on solid supports, the most important methods will be briefly described, whereas in the next sections immobilization examples will be discussed in connection with different support types.
1.2.1. Physical methods
The main physical immobilization methods are:
- non-covalent adsorption (mainly by hydrophobic and van der Waals interactions) and ionic adsorption (by ionic interactions);
- physical entrapment, by inclusion of an enzyme in a polymer network that can be either an organic polymer or a silica sol-gel, or in a membrane device such as a hollow fiber or a microcapsule.
Adsorption is the most simple and less expensive method and does not modify the active conformation of enzymes. Consequently, adsorption on the surface of a water-insoluble carrier through non-covalent binding usually does not cause significant inactivation.
However, physical binding is generally not strong enough to keep the enzyme fixed to the carrier under industrial conditions of high reactant and product concentrations and high ionic strength (Sheldon, 2007a). Such leaching could be important in aqueous reaction media, making the process design and reaction engineering more difficult (Brady and Jordaan, 2009). Despite these disadvantages, adsorption is frequently used in large scale processes, particularly where the enzyme is not expensive (Lalonde and Margolin, 2002).
Ionic binding on water-insoluble matrices holding ionic exchange groups is generally stronger than adsorption. The tightness of binding is dependent on the proximity and charge of the binding residues on the protein surface and the carrier. Protein binding can be efficient and strong if factors which affect ionization like as pH, counter-ion identity,
hydrophobicity and ionic strength are optimal (Lalonde and Margolin, 2002). However, leaking problems can still occur, particularly at high ionic strength or at pH variations. The protein is bound through association of side chains of amino acids such as aspartate and glutamate (carboxylate ions), or lysine (ammonium ions), with oppositely charged groups on the carrier. Ionic adsorption was the oldest immobilization method that has been used at industrial scale (Chibata, 1978). Ionic attachment to nonionic surfaces can also be realized through a polyvalent metal cation. Chelation of a transition metal by both the carrier surface and the enzyme results in binding to the surface (Lalonde and Margolin, 2002).
Entrapment is an irreversible method that refers to physical confinement of an enzyme in an environment where the substrate is able to penetrate but the enzyme cannot leak.
Entrapment of enzymes can occur either in polymer matrices (organic polymer or silica sol-gel) or in membrane devices (microcapsule or hollow fiber). Entrapment requires the synthesis of the polymeric network in the presence of the enzyme (Sheldon, 2007a). This type of immobilization might have several disadvantages like as high cost of immobilization, or strong mass transfer limitations. Another drawback can be a lower loading capacity than in case of other immobilization techniques.
In case of matrix entrapment, selection of the best carrier is the key issue, and an essential parameter is the ratio between the size of immobilized biomolecule and pore size of support material. In case of small pores adsorption occurs only on external surfaces, leading to low enzyme loading, while large pores could cause enzyme leaking (Górecka and Jastrzębska, 2011). Despite all these drawbacks, matrix entrapment has proven to be a particularly easy and effective way to immobilize enzymes. The relatively mild immobilization conditions help to preserve the biological function of immobilized enzymes and inactivation is usually lower than in case of chemical binding methods (Rodgers et al., 2006). Moreover, depending on the matrix density, the protein environment can be similar to that of protein in the bulk reaction media, preserving the catalytic activity during utilization (Lalonde and Margolin, 2002). In some cases, matrix entrapment allows multi- enzyme reactions, by co-immobilization of coupled enzyme systems. Co-encapsulation of a redox enzyme and a cofactor regeneration system in silica particles is such an example (Betancor et al., 2006).
The main characteristic of microencapsulation in membrane devices is that biocatalysts are restricted by the membrane walls (usually in a form of a capsule), but free-floating within the core space. The key factor of efficiency is the proper pore size of the membrane. This
limited access to the microcapsule interior is one of the main advantages, protecting the biocatalyst from harsh environmental conditions. Biocatalyst leakage does not occur and the activity is supposedly preserved during exploitation. Microencapsulation is also suitable for multi-enzyme systems for sequential enzymatic reactions. The most important disadvantage of microencapsulation is the necessity for a very strict pore size control, which is particularly difficult in the case of enzymes with small molecular weight (Górecka and Jastrzębska, 2011). This technique is not applicable for high molecular weight substrates.
1.2.2. Chemical methods
Chemical immobilization methods are covalent binding and cross-linking (Sevella, 2011).
Covalent binding is probably the most complex immobilization method, as allows a large number of attachment possibilities between supports and enzymes. The immobilization of enzymes by covalent attachment to a solid carrier involves formation of a covalent bond between amino acid side chain residues of the protein with reactive groups on the support surface (Lalonde and Margolin, 2002). Most of functional groups of proteins usually involved in covalent binding are nucleophilic amino (lysine, histidine and arginine), thiol (cysteine) and hydroxyl groups (serine, threonine and tyrosine), as well as electrophilic carboxylate groups (aspartic acid and glutamic acid). The ε-amino group of lysine is typically used for covalent attachment to the carrier. The advantages of lysine are that it is often located on the protein surface, can be found in enough high amounts in the structure of enzymes, exhibits higher reactivity than other functional groups, and provides good bond stability (Brady and Jordaan, 2009). For efficient immobilization, reactive groups of enzymes should react in mild conditions with appropriate functional groups of supports.
Usually, the available functional groups are not enough active and must be activated.
Either the solid support or the enzyme may be activated, but to limit alteration of the tertiary structure of enzyme the functional groups of the support material are activated most often. The activation may occur prior to the coupling reaction, or a bi-functional linking reagent can be used to form the bond between enzyme and support. Several activation methods will be described later, in connection with different supports utilized for immobilization. The major advantage of covalent binding is stabilization of the immobilized enzyme. Covalent attachment is the preferred method if enzyme value is high, minimal protein leaching from the support is required, or rational control of the biocatalyst
properties is desired. Due to the stronger carrier-protein linkage, the obtained heterogeneous biocatalyst can be much more stable than in case of adsorption or entrapment (Lalonde and Margolin, 2002). However, it must be noted that harsh conditions employed during covalent binding can potentially alter the enzyme conformation, lowering the enzymatic activity. Moreover, binding of the active sites of enzyme to the support may result in total loss of activity (Zhao et al., 2006).
Cross-linking means construction of three-dimensional enzyme structures by linking the enzyme molecules covalently. Cross-linking of enzyme aggregates or crystals, using a bifunctional reagent, allows preparation of carrierless macroparticles. Sheldon considered that use of a carrier inevitably leads to dilution of activity, owing to introduction of a large portion of non-catalytic ballast, which results in lower productivities. Moreover, immobilization of an enzyme on a carrier often leads to the loss of more than 50% native activity, especially at high enzyme loadings (Sheldon, 2007a). Carrier-free immobilized enzymes, such as cross-linked enzyme crystals (CLECs), and cross-linked enzyme aggregates (CLEAs) could be a solution to overcome the before-mentioned drawbacks.
The main advantages of the cross-linking immobilization method are: (i) more concentrated enzyme activity in the catalyst than in case of carrier-bound enzymes; (ii) high stability; (iii) lower production costs, as the (potentially) expensive carrier is no more needed. Main drawbacks of this method are the difficult control of aggregates size, difficult substrate accessibility to the cores of aggregates, and lack of mechanical strength of cross-linked enzyme (Zhao et al., 2006).
1.3. Supports for enzyme immobilization
Although enzymes work in biological systems in aqueous environments, the pioneering work of Klibanov demonstrated that catalytic activity can be preserved in organic or heterogeneous reaction systems (Klibanov, 1983; Zaks and Klibanov, 1985).
Consequently, immobilization techniques have been constantly improved in the last 50 years and immobilization became one of the most important issues for industrial application of biocatalytic reactions. Immobilization methods range from binding to prefabricated carrier materials to packaging in enzyme crystals or powders (Tischer and Kasche, 1999). The availability of numerous support materials and methods to carry out immobilization led to a large number of literature reports about this subject. Practically, all
enzymes used in biocatalytic applications have been tested for immobilization. Even if considerable effort was focused on cross-linking of enzyme molecules to yield immobilized enzyme aggregates (Sheldon, 2007b), the major part of the experimental investigations targeted methods which involve the presence of an insoluble support material. The properties of the immobilized enzyme are determined by the characteristics of both the enzyme and the carrier material. Therefore, the choice of support is essential to obtain an immobilized enzyme with high activity retention and increased stability, in economically viable conditions.
The selection of an appropriate support is essential for enzymatic reactions carried out in organic media, as it was demonstrated by Adlercreutz. In α-chymotrypsin catalyzed alcoholysis of N-acetyl-L-phenylalanine ethyl ester with 1-butanol, high rates were obtained with Celite as immobilization support, but only about one third of the ethyl ester was transesterified, the rest was hydrolyzed. Using a polyamide support (Accurel PA6) alcoholysis was the dominating reaction, and at low water activity (0.33) hydrolysis was completely suppressed. Controlled pore glass derivatized with hexyl groups favoured alcoholysis, while the same support derivatized with glucosyl groups favored hydrolysis, at water activities exceeding 0.8 (Adlercreutz, 1991).
The main types of support materials and their characteristics will be reviewed in this section, with emphasis on their characteristics, advantages and disadvantages.
1.3.1. Types of support materials
It is impossible to find a universal carrier for enzyme immobilization, since the specific requirements are different for every enzyme and application. Chemical and physical characteristics of carrier materials are both important to allow the selection of the most suitable support. The main chemical characteristics are chemical composition, functional groups available for interaction with the enzyme, and chemical stability in the employed reaction conditions. Among physical characteristics, several are considered important to any material used as carrier (Kennedy and Cabral, 1987):
• Appropriate surface area
• Permeability for substrates and products
• Hydrophilic character
• Insolubility in the reaction medium
• Thermal stability
• Suitable shape and particle size
Other physical characteristics of the carrier material which influence the immobilization are swelling behavior, accessible volume of the matrix, single-particle compression behavior, flow resistance (in case of fixed-bed applications) (Tischer and Kasche, 1999).
Based on their chemical composition, the supports for enzyme immobilization are usually classified as inorganic and organic materials (Kennedy and Cabral, 1987; Péter, 2005).
Worsfold recommended a different classification of supports, in three categories: (i) hydrophilic biopolymers based on natural polysaccharides; (ii) lipophylic synthetic organic polymers, and (iii) inorganic materials (Worsfold, 1995).
As it will be seen, in the last years classification of an immobilization carrier as organic or inorganic became increasingly difficult, as more and more immobilization methods employed hybrid organic-inorganic supports. In this survey the core material was considered relevant for the chemical character of the support, e.g. silica and magnetite carriers were classified as inorganic, even if they were composite materials with organic, mainly polymeric, compounds.
1.3.1.1. Inorganic carriers
Historically, the first enzyme immobilization was accomplished in 1916 by Nelson and Griffin, using Al(OH)3 and charcoal for immobilization of invertase from yeast (Nelson and Griffin, 1916). However, in the subsequent period organic supports were preferred, due to the higher reactivity and easier modification or activation of their functional groups, making them suitable for coupling with enzymes. Nowadays, the inorganic carriers, particularly the silica-based materials, are back in the attention. Considering the possible industrial applications, the inorganic supports present several advantages, as high mechanical resistance, temperature and pH stability, resistance to organic solvents and microbial attack.
Numerous inorganic matrixes have been tested as supports for immobilization of enzymes, Part of them were cheap minerals or other materials of natural origin, but synthetic products have numerous advantages and are now used in most applications. An overview of selected inorganic carriers is presented in Table 1.2. Inorganic supports have been used for immobilization by adsorption, entrapment and covalent binding techniques.
Table 1.2. Inorganic supports used for immobilization of enzymes Support type Enzyme
immobilized
Immobilization method
Reference
Natural
CaCO3 lipase adsorption Roşu et al., 1998
Pumice cellulase adsorption Pazarlioğlu et al.,
2005
Kaolinite lipase adsorption Iso et al., 2001
Clay minerals cellulase adsorption Sinegani et al.,
2005
Montmorillonite lipase adsorbtion Scherer et al., 2011
Bentonite lipase adsorption Yeşiloğlu, 2005
Synthetic
Alumina pancreatin adsorption Silvestre et al.,
2009
Activated carbon pancreatin adsorption Silvestre et al., 2009
Pumice, ZrOCl2 activated
cellulase adsorption Pazarlioğlu et al., 2005
Mesoporous molecular sieves
horeseradish peroxidase, trypsin
adsorption Deere et al., 2003 Ceramics, derivatized lipase
glucoamylase
adsorption Kamori et al., 2002 Mesoporous silica peroxidase
catalase protease
adsorption, followed by encapsulation
Wang and Caruso, 2005
Porous zirconia, 3-isothiocyanato- propyl activated
trypsin
chymotrypsin, papain, pepsin
covalent binding Huckel et al., 1996
Controlled pore glass, propylamino- derivatised
alcohol oxidase covalent bindig, glutaraldehyde activation
Azevedo et al., 2004
Ceramic, propylamino- derivatized
β-D-galactosidase covalent bindig, glutaraldehyde activation
Saito et al., 1994
Magnetic Fe3O4 glucose oxidase, chymotrypsin
covalent binding, carbodiimide activation
Koneracká et al., 2002
Magnetic Fe3O4, propylamino- derivatized
peroxidase adsorption Ma et al., 2003
Silica, propylamino- derivatized
α-amylase covalent bindig, glutaraldehyde activation
Lim et al., 2003
Silica penicillin G
acylase
covalent bindig, glutaraldehyde activation
Kheirolomoon et al., 2002
Silica sol-gel, functionalized
organophosphorous hydrolase
entrapment Lei et al., 2002
Adsorption is a simple method that usually results in preservation of catalytic activity, as the active centre is not involved in the immobilization process. Immobilization on natural inorganic supports was used mainly on empirical basis, based on the observation that a certain support has been proved efficient for immobilization of several enzymes. It is the simplest technique and probably the cheapest method to obtain immobilized biocatalysts, but also results in the highest degree of protein desorption during the biocatalytic process.
Consequently, several studies were initiated to tailor the best protein-carrier combinations, based on the physicochemical properties of the support and the surface potential of the biomolecules (Torres-Salas et al., 2011).
Among natural inorganic carriers, porous kaolinite particles were considered appropriate for immobilization of Pseudomonas fluorescens lipase (Iso et al., 2001). When used for production of biodiesels, the activity of immobilized lipase was highly increased in comparison with the free enzyme. The immobilized enzyme was used repeatedly, without troublesome method of separation and significant decrease of its activity. The authors considered that the active sites of the enzyme became more effective following immobilization, as the enzyme was dispersed on the surface of carrier particles.
A very simple natural carrier, CaCO3 has been efficiently used for immobilization of Pseudomonas lipase, to obtain a biocatalyst for transesterification of docosahexaenoic acid ethyl ester with glycerol (Roşu et al., 1998). CaCO3 particles played the role of surfactant and enzyme transporter to the glycerol-ethyl ester interface. Microscopic analysis of the emulsified reaction mixture revealed that CaCO3 particles stabilized the substrate droplets by adsorption on their surface. The reaction rate was five times higher with lipase adsorbed on fine CaCO3 powder compared to the dissolved free lipase, due to a larger interfacial area available for the enzyme action.
Although they are cheap and easily available, natural inorganic materials are not commonly employed as immobilization carriers, since their characteristics are generally not constant. Synthetic materials are more appropriate, as their properties: surface area, surface morphology, chemical composition, porosity, pore distribution, can be assessed for a specific purpose. Moreover, such supports are suitable for structural functionalization.
Numerous synthetic inorganic materials have been investigated as carriers: metals, metal oxides, porous glass, and ceramics, as results from Table 1.2.
Silvestre et al. investigated the immobilization of pancreatin on alumina and activated carbon by adsorption. Activated carbon proved to be a more efficient support, considering
both immobilization yield of the enzyme (100%, compared to 37% on alumina) and catalytic efficiency of the adsorbed biocatalyst. Pancreatin adsorbed on activated carbon was reused 5 times for hydrolysis of whey without any loose of activity, while in case of alumina only 2 hydrolysis cycles were performed at 100% activity. However, at longer utilization (up to 20 reaction cycles) the performance of the two support materials was similar, resulting in 65% and 67% residual activity, respectively (Silvestre et al., 2009).
Since first described in 1992, mesoporous molecular sieves were considered attractive candidates for a wide range of applications in catalysis, having large surface areas (up to 1000 m2g-1), highly ordered pore structures, and tight pore size distributions (Deere et al., 2003). Different adsorption techniques were investigated in connection with these types of carriers, concluding that functionalized mesoporous molecular sieves present enhanced interactions within the protein surface and the surface of the support material. Deere et al.
used two MCM-41 type mesoporous molecular sieve materials, with average pore diameters of 28 and 45Å, for adsorption immobilization of several oxidative enzymes. The proteins have penetrated the mesoporosity, but only partially travelled down through the pore channels. At high protein loadings, a substantial amount was no more accessible to the substrate and the activities declined, due to packing of protein molecules into the mesoporosity (Deere et al., 2003).
As porous silica or glass materials have disadvantages of solubility at pH above 8.0 and hydrolysis of siloxane bonds at strong acidic pH values, for enzymes requiring extreme pH values utilization of transition metals as carriers could be an alternative. Utilization of chelating properties of transition metals was investigated by several research groups.
Particularly, titanium and zirconium oxides have been demonstrated to be useful for activation of different supports, like cellulose, porous glass, or silica gel (Kennedy and Cabral, 1985). A different approach, proposed by Huckel et al., employed porous zirconia, obtained by plasma spray technology, as support material for immobilization. The advantages of zirconia-based materials are high density and extremely high chemical, thermal and pH stabilities. The surface of zirconia was hydrothermally treated and activated with 3-isothiocyanato-propyltriethoxy silane, followed by covalent attachment of enzymes to this activated support. Several proteolytic enzymes were immobilized in this way, obtaining protein coupling efficiencies in the range of 6-80 mg/g. The highest specific activity was obtained for zirconia-chymotrypsin biocatalyst, reaching 61% of activity of the free enzyme (Huckel et al., 1996).
Controlled pore glass and ceramic materials were considered for a long period among the most important inorganic supports. Controlled-pore glass has wide solvent compatibility, can be obtained in 4.5-400 nm range with narrow pore size distribution, but is unstable at pH>8, limiting its applicability (Kennedy and Cabral, 1987). Due to relative chemical inertness of inorganic support materials, which have mainly hydroxyl groups on their surface, derivatization with a silanization agent holding an organic functional group was necessary, to generate an activated support (Fig. 1.1).
O Si O
OH
Si O
OH
RO Si OR
OR
(CH2)nX
O Si O
O
Si O
O Si
Si O
(CH2)nX OR
(CH2)nX OR
H+
+
R - etoxy; X - organic functional group
Fig. 1.1. Silanization of the surface of porous glass (Kennedy and Cabral, 1987)
γ-Aminopropylsilane derivatives of porous glass were the most popular activated supports, being commercialized since the 1970’s by Corning Inc., USA, as carriers for covalent enzyme immobilization (Chibata, 1978). The organosilane derivatives of porous glass were in some cases reacted with the enzyme using a cross-linker. In other cases, the grafted organic functional groups were transformed in more reactive derivatives, able to form covalent linkages with enzymes in mild conditions.
Azevedo et al. reported immobilization of alcohol oxidase on γ-propylamino derivatized controlled pore glass by covalent attachment, using glutaraldehyde as cross-linker. The best results were obtained with porous glass beads having 100 µm diameter and 500Ǻ pore size. Using this biocatalyst in a continuous bioreactor, the initial performance was preserved for more than 14 h operation at 32°C (Azevedo et al., 2004).
Other porous inorganic carriers, like as porous ceramics or porous silica, are more suitable for industrial purposes than controlled-pore glass, on condition to have been obtained with desired pore size and activated in a proper way. Saito et al. used porous ceramic with 37.6 nm mean pore diameter and 77.7 m2/g specific surface for immobilization of a thermostable β-D-galactosidase from Escherichia coli. From several activating organosilanes, 3-[2-(2-aminoethyleminoethylamino)propyl]trimethoxysilane led to the
highest residual activity when β-D-galactosidase was immobilized on the derivatized support by glutaraldehyde cross-linking. The amount of covalently immobilized β-D- galactosidase was double, compared to the enzyme immobilized by physical adsorption on the same carrier. The specific activity of the immobilized enzyme was about 50%, related to the free enzyme (Saito et al., 1994). A different approach to immobilize enzymes on a ceramic carrier was proposed by Kamori et al. They carried out functionalization of the ceramic material by reaction with organosilanes, obtaining methacryloyloxy and amino functional groups on the porous surface. Further on, the immobilization was accomplished by adsorption on these organo-functionalized supports (Fig. 1.2). If the pore size of support and the organic functional group were appropriately selected, the activated support was able to selectively concentrate the enzyme protein from a crude enzyme preparate. Both lipase and glucoamylase immobilized by this method demonstrated high catalytic efficiency (Kamori et al., 2002).
OH OH OH (a)
ceramics surface
O O O (b) ceramics
surface Si
Si Si
(CH2)3 O C C CH3
O CH3 O
O O (c) ceramics
surface Si
Si Si
(CH2)3 NH2
Fig. 1.2. Ceramic support for immobilization of enzymes, non-functionalized (a), derivatized with 3-trimethoxysilyl(propyl) methacrylate (b), and derivatized with 3-
(triethoxysilyl)propylamine (Kamori et al., 2002)
Mesoporous silica was proved particularly efficient for immobilization, allowing to develop an immobilization strategy based on structural and functional characteristics of the enzyme (Lee et al., 2009). Ordered mesoporous silicas provide excellent opportunities for immobilization of enzymes via covalent binding, due to the availability of well defined silanol groups. These groups provide reactive sites for functionalization and offer tunable surface properties, allowing to control the position and density of the immobilized catalyst precisely. Particularly, the sol-gel technique, developed in the last decades, was able to produce a new generation of robust and efficient silica-based biocatalysts, with excellent catalytic performances (Pierre, 2004). Since most matrixes obtained by the sol-gel method and utilized for enzyme entrapment are hybrid inorganic-organic materials, they will be discussed in a separate section.
Functionalized magnetic materials
Utilization of magnetic materials instead of conventional water-insoluble supports was first reported in the 1970’s (Zaborsky, 1976). The main advantages of these carriers were the ease of separation and process control. The immobilized enzyme can be simply separated from the reaction mixture, even from viscous solutions or suspensions (Brady and Jordaan, 2009). Moreover, such biocatalysts are particularly suited for fluidized bed operations (Bahar and Celebi, 2000). Most magnetic micro- or nanoparticles used for immobilization were of core-shell type, having the biomolecules connected to the magnetic core through an organic or polymeric shell (Ma et al., 2003). They are basically non-porous supports, but may present some advantages compared to the use of porous supports, as absence of external diffusion problems. The most commonly used magnetic material was magnetite, Fe3O4, a superparamagnetic material. Various immobilization methodologies have been investigated, like as: (i) functionalization of magnetite followed by covalent binding (Koneracká et al., 2002); (ii) adsorption of enzyme on activated magnetite followed by cross-linking with glutaraldehyde (Van Leemputten and Horisberger, 1974); (iii) coating of magnetic particles with a polymeric material by polymerization (Sureshkumar and Lee, 2011) or copolymerization (Kondoi and Fukuda, 1997) in presence of magnetite, followed by covalent attachment of the enzyme. Several types of magnetic composite materials will be discussed in Section 1.3.1.2.
Covalent binding was mainly used for immobilization of enzymes on modified magnetic nanoparticles, by formation of covalent linkages between functional groups on the activated particle surface and appropriate functional groups of enzymes (particularly amino groups). This method often resulted in decrease of enzyme activity, but could achieve strong binding of enzyme on the magnetic particle. Magnetic particles were synthesized as macro ions by Koneracká et al. by the co-precipitation method, and used for immobilization of several enzymes, e.g. glucose oxidase and chymotrypsin. Covalent coupling of enzyme was accomplished with 1-[3-(dimethylamino)propyl]-3- ethylcarbodiimide hydrochloride as cross-linking agent. The presence of free hydroxyl groups on the surface of particles was responsible for binding of the protein. The maximum activity of the immobilized enzyme was reached by performing the coupling reaction at pH 4.5 (Koneracká et al., 2002). Another carbodiimide derivative, 1-ethyl-3-(3- dimethylaminopropyl) carbodiimide, was studied as activating agent for covalent
immobilization of lipase on Fe3O4 magnetic nanoparticles. The obtained biocatalyst was successfully used for production of biodiesel, as the immobilized lipase retained about 75% of the initial activity and was suitable for recycling (Xie and Ma, 2010). In a different approach, magnetic Fe3O4 nanoparticles were prepared by the chemical coprecipitation method and subsequently coated with 3-aminopropyltriethoxysilane, to achieve amino- functionalized magnetic nanoparticles. The coupling agent for covalent attachment of lipase to the surface of activated nanoparticle was glutaraldehyde, as presented in Fig. 1.3.
The activity recovery yield of Serratia marcescens lipase was up to 62% (Hu et al., 2009).
Fig. 1.3. Reaction scheme of covalent immobilization of lipase on amino-functionalized Fe3O4 nanoparticles (Hu et al., 2009)
Although covalent binding was obviously the most popular method for immobilization on coated or composite magnetic particles, it must be pointed out that adsorption interactions between the organic coatings covering the surface of particles and the appropriate functional groups of the enzymes can not be excluded. Moreover, it was reported the attachment of horseradish peroxidase on such a derivatized magnetic support only by adsorption. 3-Aminopropyl triethoxysilane was used to coat the surface of the Fe3O4 nanoparticles with a near monolayer of amino silane (67% coverage ratio), and the resulting amino active groups were able to adsorb biomolecules (Ma et al., 2003).
Magnetic nanoparticles
Nanotechnology-inspired biocatalyst systems were introduced only in the recent years, the first reports dating from the late 1980’s (Wang, 2006). Enzyme molecules are in order of nanometers, making them possible to benefit from the high surface area-to-volume ratio of nanomaterials. Carbon nanotubes, superparamagnetic nanoparticles, and mesoporous materials represent the most important classes of nano-sized matrices. However,
nanoparticles as such have two disadvantages when used as carriers of enzymes: (i) they often form aggregates and ultrasonication has to be carried out for temporary dispersion;
(ii) because their size, separation by either centrifugation or membranes is not simple.
Superparamagnetism can solve both these problems. Superparamagnetic materials become magnetic only in the presence of a magnetic field. Fortunately, magnetic particles with size less than 30 nm show superparamagnetism, meaning that they disperse easily in solution and can be recovered by use of a simple magnet (Gupta et al., 2011).
Most studies with nanoparticles have been dedicated to improvement of enzyme activity and loading, rather than to enzyme stabilization. However, good enzyme stabilization was reported with covalently attached lipase on magnetic γ-Fe2O3 nanoparticles. The surface of nanoparticles was functionalized by Dyal et al. with 11-bromoundecanoic acid, and the resulted intermediate was reacted with 2-thiophene thiolate, to give thiophene- functionalized nanoparticles. These have been acetylated with acetic anhydride, or were reacted with nitrosonium tetrafluoroborate, to produce the appropriate nitroso derivative.
The acetylated nanoparticles were reacted directly with the enzyme, which was chemically bonded to the nanoparticle surface via a C=N bond. The nitroso-functionalized nanoparticles were reduced with SnCl2, and the enzyme was chemically bonded to the resulted amine-functionalized nanoparticles by glutaraldehyde. It must be noticed that coating with 11-bromoundecanoic acid decreased the magnetization of γ-Fe2O3 only by 12%. The enzyme immobilized on acetylated nanoparticles demonstrated long-term stability, with only 15% decrease of activity over one month (Dyal et al., 2003).
1.3.1.2. Organic carriers
Even having some disadvantages compared to inorganic matrices, like lower stability at high temperatures, organic solvents or microbial attack, the major part of supports already employed for large-scale applications are organic materials. The major argument for utilization of organic carriers for enzyme immobilization is the variety of functional organic groups which can be attached, making possible numerous options to bind the enzyme, particularly by covalent linkages. Typically, organic support materials are of polymeric nature and can be classified as natural macromolecules or synthetic polymers (Kennedy and Cabral, 1987).


![Fig. 3.6. Effect of Tween 80 concentration on the particle size in 40% paraffin oil and 60% n-octane Tween 80 concentration [%]1234 50.00.20.40.60.81.01.21.4200.0400.0600.0Span Mean size [µµµ µm]](https://thumb-eu.123doks.com/thumbv2/9dokorg/872161.46904/93.892.243.678.117.441/effect-tween-concentration-particle-paraffin-octane-tween-concentration.webp)